乙酰溴-Alpha-D-葡萄糖酮酸甲基酯
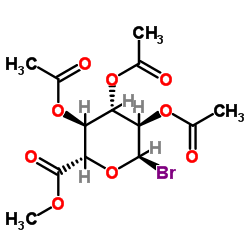
乙酰溴-Alpha-D-葡萄糖酮酸甲基酯结构式

|
常用名 | 乙酰溴-Alpha-D-葡萄糖酮酸甲基酯 | 英文名 | Methyl acetobromo-α-D-glucuronate |
|---|---|---|---|---|
| CAS号 | 21085-72-3 | 分子量 | 397.173 | |
| 密度 | 1.5±0.1 g/cm3 | 沸点 | 388.6±42.0 °C at 760 mmHg | |
| 分子式 | C13H17BrO9 | 熔点 | 80-110 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 188.8±27.9 °C |
| 中文名 | 乙酰溴-Alpha-D-葡萄糖酮酸甲基酯 |
|---|---|
| 英文名 | Acetobromo-α-D-glucuronic Acid Methyl Ester |
| 中文别名 | 乙酰溴-alpha-D-葡萄糖醛酸甲酯 | Α-D -葡萄糖醛酸甲基酯 | 乙酰溴-α-D-葡萄糖醛酸甲酯 | 1-溴-1-脱氧-2,3,4-三-O-乙酰基-ALPHA-D-葡萄糖醛酸甲酯 | 乙酰溴-Α-D-葡萄糖酮酸甲基酯 |
| 英文别名 | 更多 |
| 密度 | 1.5±0.1 g/cm3 |
|---|---|
| 沸点 | 388.6±42.0 °C at 760 mmHg |
| 熔点 | 80-110 °C(lit.) |
| 分子式 | C13H17BrO9 |
| 分子量 | 397.173 |
| 闪点 | 188.8±27.9 °C |
| 精确质量 | 396.005585 |
| PSA | 114.43000 |
| LogP | 2.20 |
| InChIKey | GWTNLHGTLIBHHZ-YAXHFPQCSA-N |
| SMILES | COC(=O)C1OC(Br)C(OC(C)=O)C(OC(C)=O)C1OC(C)=O |
| 外观性状 | 白色结晶固体 |
| 蒸汽压 | 0.0±0.9 mmHg at 25°C |
| 折射率 | 1.505 |
| 储存条件 | -20°C冷藏、密闭、干燥处 |
| 稳定性 | 按规定使用和贮存的不会分解,避氧化物 |
| 更多 | 1. 性状:白色结晶 2. 密度(g/mL25 ºC):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):80-110 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,3hPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(mmHg,20 ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(正辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xi |
| 安全声明 (欧洲) | S22-S24/25 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| 海关编码 | 2932999099 |
| 乙酰溴-Alpha-D-葡萄糖酮酸甲基酯上游产品 9 | |
|---|---|
| 乙酰溴-Alpha-D-葡萄糖酮酸甲基酯下游产品 10 | |
| 海关编码 | 2932999099 |
|---|---|
| 中文概述 | 2932999099. 其他仅含氧杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Mitochondrial KATP channel inhibition blunts arrhythmia protection in ischemic exercised hearts.
Am. J. Physiol. Heart Circ. Physiol. 299 , H175-183, (2010) The mechanisms responsible for anti-arrhythmic protection during ischemia-reperfusion (IR) in exercised hearts are not fully understood. The purpose of this investigation was to examine whether the AT... |
|
|
Glucuronidation of HMR1098 in human microsomes: evidence for the involvement of UGT1A1 in the formation of S-glucuronides.
Drug Metab. Dispos. 31 , 1027-1034, (2003) HMR1098, a novel KATP-blocking agent, is metabolized to form an S-glucuronide in rat and dog bile. Synthesis of the S-glucuronide metabolite was studied in human liver and kidney microsomes. Recombina... |
|
|
Single channel studies of the ATP-regulated potassium channel in brain mitochondria.
J. Bioenerg. Biomembr. 41 , 323-334, (2009) Mitochondrial potassium channels in the brain have been suggested to have an important role in neuroprotection. The single channel activity of mitochondrial potassium channels was measured after recon... |
| Acetobromo-α-D-glucuronic acid, methyl ester |
| Methyl 2,3,4-tri-O-acetyl-α-D-glucopyranosyluronate bromide |
| Methyl acetobromo-α-D-glucuronate |
| Acetobromo-α-D-glucuronic acid methyl ester |
| EINECS 244-203-4 |
| (2R,3R,4S,5S,6S)-2-Bromo-6-(methoxycarbonyl)tetrahydro-2H-pyran-3,4,5-triyl triacetate |
| Acetobromo-alpha-D-glucuronic acid methyl ester |
| α-D-Glucopyranuronic acid, 1-bromo-1-deoxy-, methyl ester, triacetate |
| α-D-Glucopyranuronosyl bromide, methyl ester, triacetate |
| MFCD00061613 |
 CAS号7355-18-2
CAS号7355-18-2 CAS号3082-96-0
CAS号3082-96-0 CAS号72692-06-9
CAS号72692-06-9 CAS号5432-32-6
CAS号5432-32-6 CAS号82228-14-6
CAS号82228-14-6 CAS号108-24-7
CAS号108-24-7![(1S,2R,3R,5S)-2,3,6-trihydroxy-4,8-dioxabicyclo[3.3.0]octan-7-one结构式](https://image.chemsrc.com/caspic/144/63-29-6.png) CAS号63-29-6
CAS号63-29-6 CAS号186581-53-3
CAS号186581-53-3 CAS号77668-10-1
CAS号77668-10-1 CAS号34096-83-8
CAS号34096-83-8 CAS号14364-66-0
CAS号14364-66-0 CAS号120858-07-3
CAS号120858-07-3 CAS号1852-49-9
CAS号1852-49-9 CAS号20290-09-9
CAS号20290-09-9 CAS号1852-44-4
CAS号1852-44-4 CAS号26399-82-6
CAS号26399-82-6 CAS号29587-10-8
CAS号29587-10-8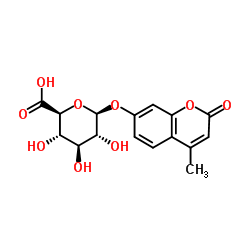 CAS号6160-80-1
CAS号6160-80-1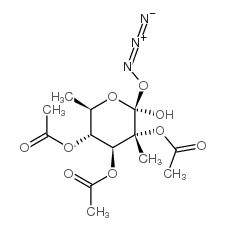 CAS号67776-38-9
CAS号67776-38-9
