α-甲基-D-半乳糖苷
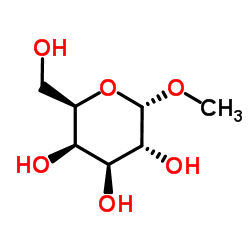
α-甲基-D-半乳糖苷结构式

|
常用名 | α-甲基-D-半乳糖苷 | 英文名 | Methyl α-D-mannopyranoside |
|---|---|---|---|---|
| CAS号 | 3396-99-4 | 分子量 | 194.182 | |
| 密度 | 1.5±0.1 g/cm3 | 沸点 | 389.1±42.0 °C at 760 mmHg | |
| 分子式 | C7H14O6 | 熔点 | 116-117 °C(lit.) | |
| MSDS | 中文版 美版 | 闪点 | 189.1±27.9 °C |
α-甲基-D-半乳糖苷用途用作制药中间体 |
| 中文名 | Α-D-乳酸吡喃糖苷甲酯 |
|---|---|
| 英文名 | methyl α-D-galactoside |
| 中文别名 | Alpha-D-乳酸吡喃糖苷甲酯单水合物 | 甲基-α-D-半乳糖苷 | alpha-D-乳酸吡喃糖苷甲酯 | 甲基α-D-吡喃半乳糖苷一水合物 | α-D-乳酸吡喃糖苷甲酯 | 甲基-Alpha-D-半乳糖苷 | α-甲基-D-半乳糖苷 |
| 英文别名 | 更多 |
| 密度 | 1.5±0.1 g/cm3 |
|---|---|
| 沸点 | 389.1±42.0 °C at 760 mmHg |
| 熔点 | 116-117 °C(lit.) |
| 分子式 | C7H14O6 |
| 分子量 | 194.182 |
| 闪点 | 189.1±27.9 °C |
| 精确质量 | 194.079041 |
| PSA | 99.38000 |
| LogP | -2.69 |
| InChIKey | HOVAGTYPODGVJG-PZRMXXKTSA-N |
| SMILES | COC1OC(CO)C(O)C(O)C1O |
| 外观性状 | 白色或灰白色粉末 |
| 蒸汽压 | 0.0±2.0 mmHg at 25°C |
| 折射率 | 1.548 |
| 储存条件 | 常温, 避光,通风干燥处,密封保存 |
| 稳定性 | 常温常压下稳定,白色或几乎白色粉末。。 |
| 分子结构 | 1、 摩尔折射率:41.92 2、 摩尔体积(m3/mol):131.9 3、 等张比容(90.2K)379.2 4、 表面张力(dyne/cm):68.3 5、 极化率(10-24cm3):16.61 |
| 计算化学 | 1.疏水参数计算参考值(XlogP):无 2.氢键供体数量:4 3.氢键受体数量:6 4.可旋转化学键数量:2 5.互变异构体数量:无 6.拓扑分子极性表面积99.4 7.重原子数量:13 8.表面电荷:0 9.复杂度:163 10.同位素原子数量:0 11.确定原子立构中心数量:5 12.不确定原子立构中心数量:0 13.确定化学键立构中心数量:0 14.不确定化学键立构中心数量:0 15.共价键单元数量:1 |
| 更多 | 1. 性状:奶油色或白色晶体状粉末 2. 密度(g/mL,25/4℃):未确定 3. 相对蒸汽密度(g/mL,空气=1):未确定 4. 熔点(ºC):105-108 5. 沸点(ºC,常压):未确定 6. 沸点(ºC,5.2kPa):未确定 7. 折射率:未确定 8. 闪点(ºC):未确定 9. 比旋光度(º):未确定 10. 自燃点或引燃温度(ºC):未确定 11. 蒸气压(kPa,25ºC):未确定 12. 饱和蒸气压(kPa,60ºC):未确定 13. 燃烧热(KJ/mol):未确定 14. 临界温度(ºC):未确定 15. 临界压力(KPa):未确定 16. 油水(辛醇/水)分配系数的对数值:未确定 17. 爆炸上限(%,V/V):未确定 18. 爆炸下限(%,V/V):未确定 19. 溶解性:未确定 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 安全声明 (欧洲) | S24/25 |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| 海关编码 | 2932999099 |
| α-甲基-D-半乳糖苷上游产品 0 | |
|---|---|
| α-甲基-D-半乳糖苷下游产品 4 | |
| 海关编码 | 2932999099 |
|---|---|
| 中文概述 | 2932999099. 其他仅含氧杂原子的杂环化合物. 增值税率:17.0%. 退税率:13.0%. 监管条件:无. 最惠国关税:6.5%. 普通关税:20.0% |
| 申报要素 | 品名, 成分含量, 用途 |
| Summary | 2932999099. other heterocyclic compounds with oxygen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Metal-mediated allylation of enzymatically oxidized methyl α-d-galactopyranoside
Carbohydr. Res. 345 , 2610-5, (2010) The C-6 unit of methyl α-D-galactopyranoside was selectively modified by combining enzymatic oxidation with an indium-mediated allylation reaction. The Barbier-Grignard type reaction, where a carbonyl... |
|
|
alpha-Glucosidase inhibitory activity of triterpenoids from Cichorium intybus.
J. Nat. Prod. 71 , 910-3, (2008) Two new triterpenoids, 18alpha,19beta-20(30)-taraxasten-3beta,21alpha-diol (cichoridiol) (1) and 17-epi-methyl-6-hydroxyangolensate (intybusoloid) (2), were obtained from the methanolic extract of see... |
|
|
1-O-Acetyl-beta-D-galactopyranose: a novel substrate for the transglycosylation reaction catalyzed by the beta-galactosidase from Penicillium sp.
Carbohydr. Res. 337(7) , 635-42, (2002) 1-O-Acetyl-beta-D-galactopyranose (AcGal), a new substrate for beta-galactosidase, was synthesized in a stereoselective manner by the trichloroacetimidate procedure. Kinetic parameters (K(M) and k(cat... |
| Methyl-alpha-D-galactopyranoside |
| MEHTYL-A-D-GALACTOPYRANOSIDE |
| METHYL-α-D-GALACTOPYRANOSIDE |
| MFCD00064085 |
| EINECS 222-251-7 |
| Methyl α-D-galactoside |
| Methyl α-D-galactopyranoside |
| (2R,3R,4S,5R,6S)-2-(Hydroxymethyl)-6-methoxytetrahydro-2H-pyran-3,4,5-triol |
| 1-O-METHYL-GALACTOSIDE |
| methyl alpha-D-galactoside |
| Methyl α-D-mannopyranoside |
| Methyl α-D-Galactopyranoside Monohydrate |
| α-D-Mannopyranoside, methyl |
| α-D-Galactopyranoside, methyl |
| METHYL-A-D-GALACTOPYRANOSIDE |

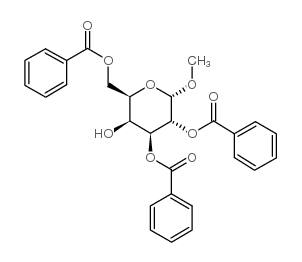 CAS号3601-36-3
CAS号3601-36-3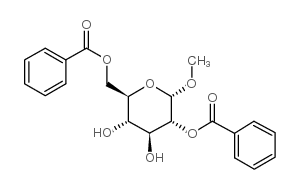 CAS号26927-44-6
CAS号26927-44-6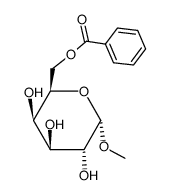 CAS号42927-28-6
CAS号42927-28-6 CAS号40269-01-0
CAS号40269-01-0





