Boc-L-丙氨醛
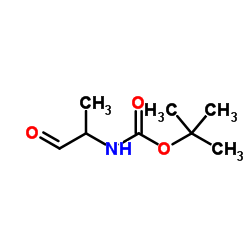
Boc-L-丙氨醛结构式

|
常用名 | Boc-L-丙氨醛 | 英文名 | Boc-L-alaninal |
|---|---|---|---|---|
| CAS号 | 79069-50-4 | 分子量 | 173.210 | |
| 密度 | 1.0±0.1 g/cm3 | 沸点 | 248.5±23.0 °C at 760 mmHg | |
| 分子式 | C8H15NO3 | 熔点 | 89 °C | |
| MSDS | 美版 | 闪点 | 104.1±22.6 °C |
| 中文名 | Boc-L-丙氨醛 |
|---|---|
| 英文名 | tert-butyl N-[(2S)-1-oxopropan-2-yl]carbamate |
| 中文别名 | N-叔丁氧羰基-L-丙氨醛 |
| 英文别名 | 更多 |
| 密度 | 1.0±0.1 g/cm3 |
|---|---|
| 沸点 | 248.5±23.0 °C at 760 mmHg |
| 熔点 | 89 °C |
| 分子式 | C8H15NO3 |
| 分子量 | 173.210 |
| 闪点 | 104.1±22.6 °C |
| 精确质量 | 173.105194 |
| PSA | 55.40000 |
| LogP | 1.23 |
| InChIKey | OEQRZPWMXXJEKU-LURJTMIESA-N |
| SMILES | CC(C=O)NC(=O)OC(C)(C)C |
| 外观性状 | Powder | White to yellow |
| 蒸汽压 | 0.0±0.5 mmHg at 25°C |
| 折射率 | 1.436 |
| 储存条件 | −20°C |
| 水溶解性 | 可溶于:甲醇 |
| 个人防护装备 | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| 危害码 (欧洲) | Xi |
| 危险品运输编码 | NONH for all modes of transport |
| WGK德国 | 3 |
| Boc-L-丙氨醛上游产品 9 | |
|---|---|
| Boc-L-丙氨醛下游产品 8 | |
|
Synthesis of the C(26)-C(32) Oxazole Fragment of Calyculin C: A Test Case for Oxazole Syntheses.
J. Org. Chem. 63 , 92-98, (1998) The synthesis of the C(26)-C(32) oxazole fragment 4 and its C(32) epimer 20 of serine/threonine protein phosphatase PP1 and PP2A inhibitor calyculin C is presented. The syn methyl arrangement in 4 was... |
|
|
Synthesis of a C20-C26 segment of superstolide A by addition of a chiral allenylzinc reagent to (R)-N-boc alaninal.
Org. Lett. 7 , 1593-1596, (2005) [reaction: see text] Additions of chiral allenylzinc and indium reagents to N-Boc alaninal were examined as a possible route to a C20-C26 segment of superstolide A. Allenylzinc reagents, prepared in s... |
|
|
Practical syntheses of a CXCR3 antagonist.
J. Org. Chem. 76 , 1767-1774, (2011) Two new, reliable syntheses of a pyrido[2,3-d]-pyrimidine inhibitor of the CXCR3 receptor are described. A nine-step synthesis of the CXCR3 inhibitor (1) from 2-aminonicotinic acid was demonstrated on... |
| tert-Butyl [(2S)-1-oxopropan-2-yl]carbamate |
| Boc-L-alanine aldehyde |
| (S)-tert-Butyl (1-oxopropan-2-yl)carbamate |
| Carbamic acid, N-[(1S)-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester |
| Boc-alaninal |
| Boc-Ala-Aldehyde |
| 2-Methyl-2-propanyl (1-oxo-2-propanyl)carbamate |
| N-T-BOC-L-ALANINAL |
| 2-Methyl-2-propanyl [(2S)-1-oxo-2-propanyl]carbamate |
| N-Boc-L-alaninal |
| Boc-L-alaninal |
| boc-L-alanine |
| Carbamic acid, N-(1-methyl-2-oxoethyl)-, 1,1-dimethylethyl ester |
| Boc-Ala-H |
 CAS号146553-06-2
CAS号146553-06-2 CAS号28875-17-4
CAS号28875-17-4 CAS号79069-13-9
CAS号79069-13-9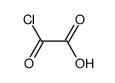 CAS号4732-69-8
CAS号4732-69-8 CAS号51814-53-0
CAS号51814-53-0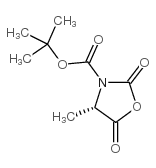 CAS号125814-30-4
CAS号125814-30-4![Carbamic acid, [(1S)-2-fluoro-1-methyl-2-oxoethyl]-, 1,1-dimethylethyl ester结构式](https://image.chemsrc.com/caspic/472/133039-96-0.png) CAS号133039-96-0
CAS号133039-96-0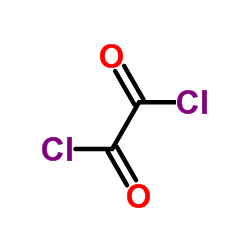 CAS号79-37-8
CAS号79-37-8![Carbamic acid, [(1S)-1-methyl-2-propenyl]-, 1,1-dimethylethyl ester (9CI)结构式](https://image.chemsrc.com/caspic/210/115378-33-1.png) CAS号115378-33-1
CAS号115378-33-1![(2E,4s)-4-[[(1,1-二甲基乙氧基)羰基]氨基]-2-戊烯酸结构式](https://image.chemsrc.com/caspic/311/142723-69-1.png) CAS号142723-69-1
CAS号142723-69-1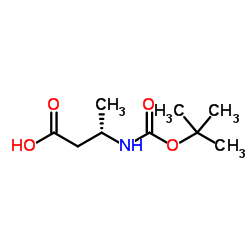 CAS号158851-30-0
CAS号158851-30-0![N-[(1S)-1-氰基乙基]-氨基甲酸叔丁酯结构式](https://image.chemsrc.com/caspic/124/130013-83-1.png) CAS号130013-83-1
CAS号130013-83-1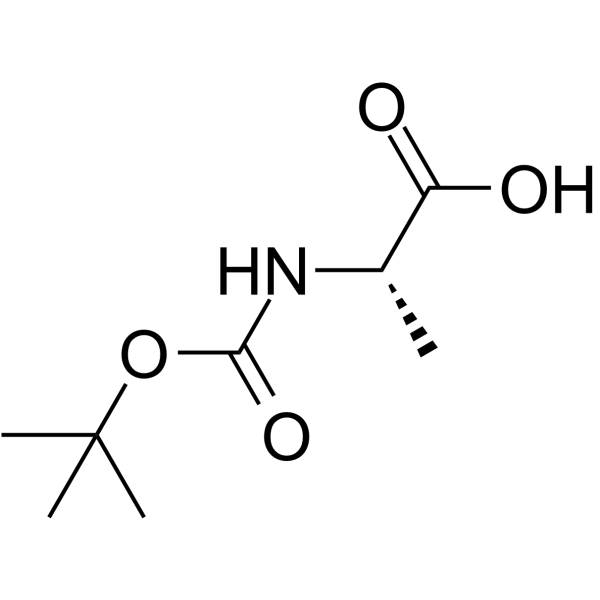 CAS号15761-38-3
CAS号15761-38-3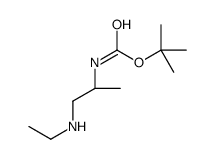 CAS号869901-70-2
CAS号869901-70-2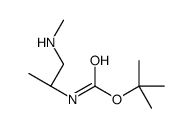 CAS号873221-70-6
CAS号873221-70-6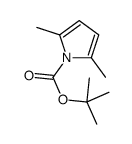 CAS号50585-36-9
CAS号50585-36-9





