|
~75% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~74% |
|
~% |
|
~% |
|
~98% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
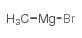
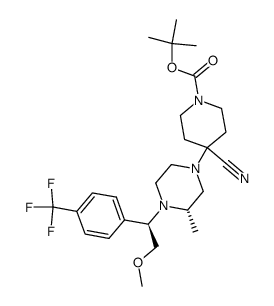
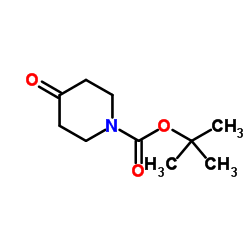
![2-Methoxy-1-[4-(trifluoromethyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/249/26771-69-7.png)
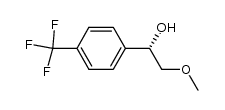
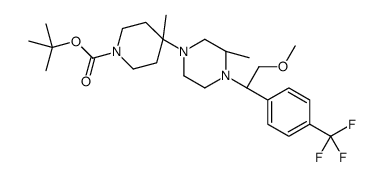
![(s)-1-[(r)-2-methoxy-1-(4-trifluoromethyl-phenyl)-ethyl]-2-methyl-piperazine Structure](https://image.chemsrc.com/caspic/369/612494-07-2.png)
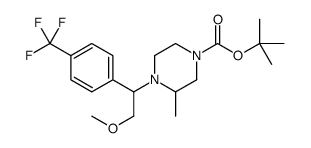
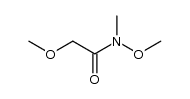
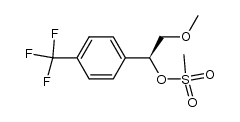
![(S)-1-[(R)-2-METHOXY-1-(4-TRIFLUOROMETHYL-PHENYL)-ETHYL]-2-METHYL-4-(4-METHYL-PIPERIDIN-4-YL)-PIPERAZINE Structure](https://image.chemsrc.com/caspic/310/696601-20-4.png)