|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~85% |
|
~28% |
|
~% |
|
~% |
|
~3% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
![2-(2-bromomethyl-4-methoxyphenyl)-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane Structure](https://image.chemsrc.com/caspic/418/1447933-44-9.png)
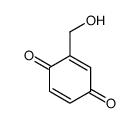

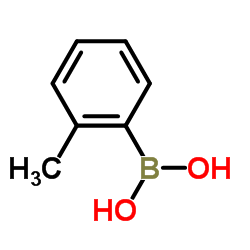
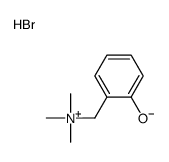
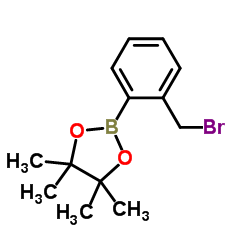
![4,4,5,5-TETRAMETHYL-[1,3,2]DIOXABOROLAN-2-OL Structure](https://image.chemsrc.com/caspic/146/25240-59-9.png)

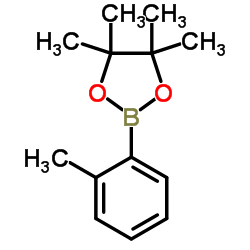
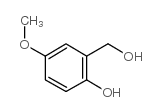
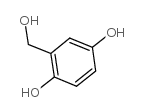
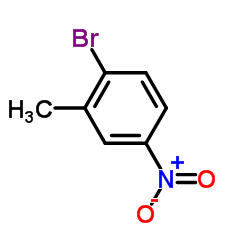
![2-(2-bromomethyl-4-nitrophenyl)-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane Structure](https://image.chemsrc.com/caspic/355/1030832-68-8.png)

![2-(2-bromomethyl-4-methylphenyl)-4,4,5,5-tetramethyl-[1,3,2]dioxaborolane Structure](https://image.chemsrc.com/caspic/456/1447933-43-8.png)

![trimethyl-[2-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-5-methoxybenzyl]-quaternaryammonium bromide Structure](https://image.chemsrc.com/caspic/294/1447933-47-2.png)
![trimethyl-[2-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-5-fluorobenzyl]-quaternaryammonium bromide Structure](https://image.chemsrc.com/caspic/218/1447933-49-4.png)