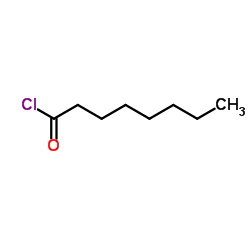
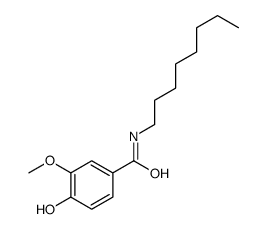
|
~94% |
|
~96% |
|
~98% |
|
~94% |
|
~90% |
|
~96% |
|
~95% |
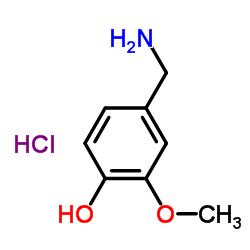
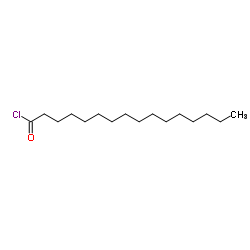
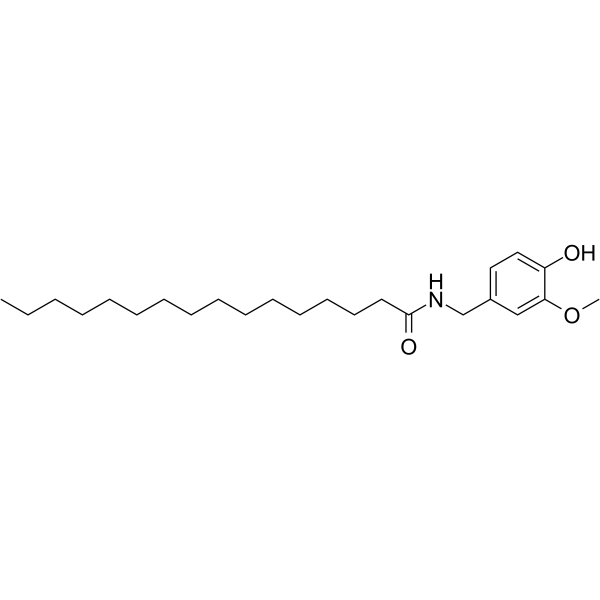
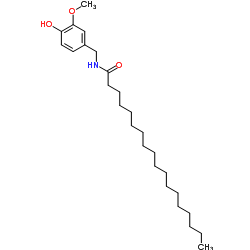

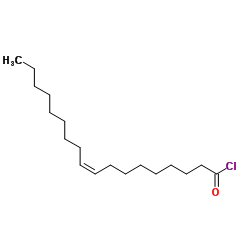


![N-[(4-hydroxy-3-methoxyphenyl)methyl]butanamide Structure](https://image.chemsrc.com/caspic/207/89575-11-1.png)
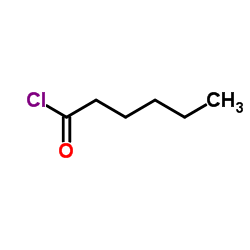
![N-[(4-hydroxy-3-methoxyphenyl)methyl]hexanamide Structure](https://image.chemsrc.com/caspic/155/58570-67-5.png)