4-Hydroxy-3-methoxybenzylamine hydrochloride
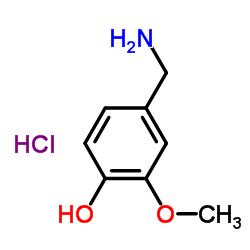
4-Hydroxy-3-methoxybenzylamine hydrochloride structure
|
Common Name | 4-Hydroxy-3-methoxybenzylamine hydrochloride | ||
|---|---|---|---|---|
| CAS Number | 7149-10-2 | Molecular Weight | 189.64 | |
| Density | N/A | Boiling Point | 292.9ºC at 760 mmHg | |
| Molecular Formula | C8H12ClNO2 | Melting Point | 219-221 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 130.9ºC | |
| Symbol |

GHS07 |
Signal Word | Warning | |
Use of 4-Hydroxy-3-methoxybenzylamine hydrochlorideVanillylamine hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Hydroxy-3-methoxybenzylamine hydrochloride |
|---|---|
| Synonym | More Synonyms |
| Description | Vanillylamine hydrochloride is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Boiling Point | 292.9ºC at 760 mmHg |
|---|---|
| Melting Point | 219-221 °C (dec.)(lit.) |
| Molecular Formula | C8H12ClNO2 |
| Molecular Weight | 189.64 |
| Flash Point | 130.9ºC |
| Exact Mass | 189.055649 |
| PSA | 55.48000 |
| LogP | 2.36180 |
| Storage condition | Refrigerator |
| Symbol |

GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H315-H319-H335 |
| Precautionary Statements | P261-P305 + P351 + P338 |
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2942000000 |
|
~98% 
4-Hydroxy-3-met... CAS#:7149-10-2 |
| Literature: Liu, Hefang; Lepoittevin, Benedicte; Roddier, Celine; Guerineau, Vincent; Bech, Loic; Herry, Jean-Marie; Bellon-Fontaine, Marie-Noelle; Roger, Philippe Polymer, 2011 , vol. 52, # 9 p. 1908 - 1916 |
|
~% 
4-Hydroxy-3-met... CAS#:7149-10-2 |
| Literature: Journal of Organic Chemistry, , vol. 53, # 5 p. 1064 - 1071 |
|
~% 
4-Hydroxy-3-met... CAS#:7149-10-2 |
| Literature: Journal of Organic Chemistry, , vol. 53, # 5 p. 1064 - 1071 |
| Precursor 3 | |
|---|---|
| DownStream 10 | |
| HS Code | 2922509090 |
|---|---|
| Summary | 2922509090. other amino-alcohol-phenols, amino-acid-phenols and other amino-compounds with oxygen function. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Inhibition by capsaicin and its related vanilloids of compound action potentials in frog sciatic nerves.
Life Sci. 92(6-7) , 368-78, (2013) Although capsaicin not only activates transient receptor potential vanilloid-1 (TRPV1) channels but also inhibits nerve conduction, the latter action has not yet been fully examined. The purpose of th... |
|
|
Synthesis of stable isotope-labeled precursors for the biosyntheses of capsaicinoids, capsinoids, and capsiconinoids.
Biosci. Biotechnol. Biochem. 75(8) , 1611-4, (2011) Stable isotope-labeled precursors were synthesized for an analysis by liquid chromatography-tandem mass spectrometry (LC-MS/MS) to elucidate the biosynthetic flow of capsaicinoids, capsinoids, and cap... |
|
|
Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuum cv. CH-19 Sweet.
Plant J. 59(6) , 953-61, (2009) Capsaicinoids are responsible for the spicy flavor of pungent peppers (Capsicum). The cultivar CH-19 Sweet is a non-pungent pepper mutant derived from a pungent pepper strain, Capsicum annuum CH-19. C... |
| EINECS 230-468-3 |
| 4-(Aminomethyl)-2-methoxyphenol hydrochloride (1:1) |
| Phenol, 4-(aminomethyl)-2-methoxy-, hydrochloride (1:1) |
| MFCD00012864 |
| 4-Hydroxy-3-methoxybenzylamine hydrochloride |
| 4-(aminomethyl)-2-methoxyphenol,hydrochloride |

![2-methoxy-4-[(methoxyimino)methyl]phenol 4-Hydroxy-3-methoxybenzaldehyde O-Methyloxime structure](https://image.chemsrc.com/caspic/259/93249-67-3.png)
 CAS#:2444-46-4
CAS#:2444-46-4 CAS#:31078-36-1
CAS#:31078-36-1 CAS#:128007-31-8
CAS#:128007-31-8 CAS#:19408-84-5
CAS#:19408-84-5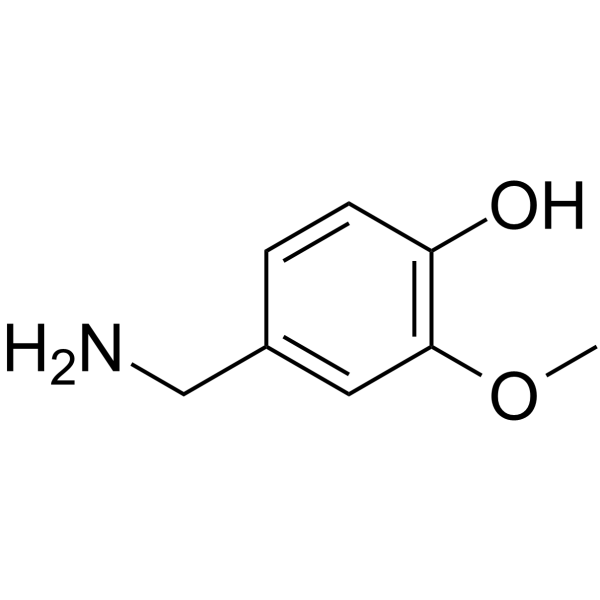 CAS#:1196-92-5
CAS#:1196-92-5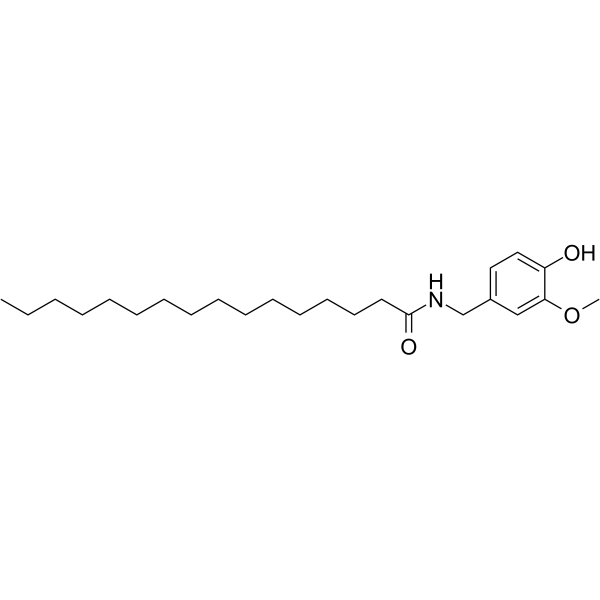 CAS#:69693-13-6
CAS#:69693-13-6![N-[(4-hydroxy-3-methoxyphenyl)methyl]prop-2-enamide structure](https://image.chemsrc.com/caspic/010/852923-26-3.png) CAS#:852923-26-3
CAS#:852923-26-3![N-[(4-hydroxy-3-methoxy-phenyl)methyl]heptanamide structure](https://image.chemsrc.com/caspic/245/89575-10-0.png) CAS#:89575-10-0
CAS#:89575-10-0![N-[(4-hydroxy-3-methoxyphenyl)methyl]butanamide structure](https://image.chemsrc.com/caspic/207/89575-11-1.png) CAS#:89575-11-1
CAS#:89575-11-1
