Thermodynamic and kinetic hydricities of metal-free hydrides
Stefan Ilic, Abdulaziz Alherz, Charles B. Musgrave, Ksenija D. Glusac
Index: 10.1039/C7CS00171A
Full Text: HTML
Abstract
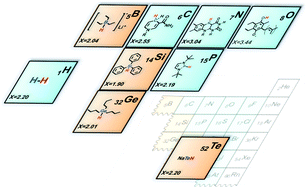
Metal-free hydrides are of increasing research interest due to their roles in recent scientific advances in catalysis, such as hydrogen activation with frustrated Lewis pairs and electrocatalytic CO2 reduction with pyridinium and other aromatic cations. The structural design of hydrides for specific applications necessitates the correct description of their thermodynamic and kinetic prowess using reliable parameters – thermodynamic hydricity (ΔGH−) and nucleophilicity (N). This review summarizes reported experimental and calculated hydricity values for more than 200 metal-free hydride donors, including carbon-, boron-, nitrogen- and silicon-based hydrides. We describe different experimental and computational methods used to obtain these thermodynamic and kinetic parameters. Furthermore, tabulated data on metal-free hydrides are discussed in terms of structure–property relationships, relevance to catalysis and contemporary limitations for replacing transition-metal hydride catalysts. Finally, several selected applications of metal-free hydrides in catalysis are described, including photosynthetic CO2 reduction and hydrogen activation with frustrated Lewis pairs.
|
Structure-based design of targeted covalent inhibitors
2018-04-05 [10.1039/C7CS00220C] |
|
Recent advances in radical-based C–N bond formation via phot...
2018-04-05 [10.1039/C7CS00572E] |
|
Correction: Spotting the differences in two-dimensional mate...
2018-04-04 [10.1039/C8CS90042F] |
|
Wearable and flexible electronics for continuous molecular m...
2018-04-03 [10.1039/C7CS00730B] |
|
Multimetallic nanosheets: synthesis and applications in fuel...
2018-04-03 [10.1039/C8CS00113H] |