Reactions of secondary propargylamines with heteroallenes for the synthesis of diverse heterocycles
Vsevolod A. Peshkov, Olga P. Pereshivko, Anton A. Nechaev, Anatoly A. Peshkov, Erik V. Van der Eycken
Index: 10.1039/C7CS00065K
Full Text: HTML
Abstract
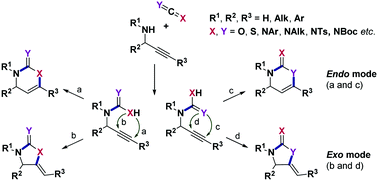
This focused review aims to summarize recent developments in the processes involving additions of secondary propargylamines to various heteroallenes and subsequent transition metal-catalyzed or electrophile-mediated cyclizations. The utility of this convenient and tunable strategy spans from the carbon dioxide fixation and target-oriented synthesis of complex natural and biologically active products to the generation of extended synthetic libraries of diverse oxygen-, nitrogen- and sulfur-containing heterocycles. For comparative purposes, the analogous transformations of propargylic alcohols are also highlighted in this account.
|
Structure-based design of targeted covalent inhibitors
2018-04-05 [10.1039/C7CS00220C] |
|
Recent advances in radical-based C–N bond formation via phot...
2018-04-05 [10.1039/C7CS00572E] |
|
Correction: Spotting the differences in two-dimensional mate...
2018-04-04 [10.1039/C8CS90042F] |
|
Wearable and flexible electronics for continuous molecular m...
2018-04-03 [10.1039/C7CS00730B] |
|
Multimetallic nanosheets: synthesis and applications in fuel...
2018-04-03 [10.1039/C8CS00113H] |