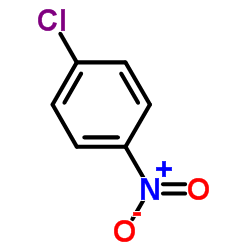
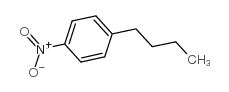
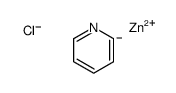
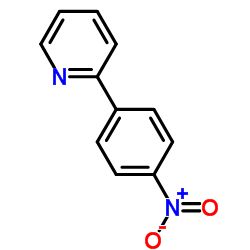
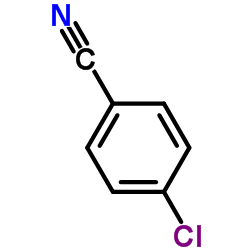
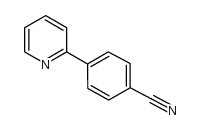
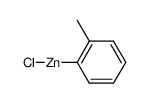
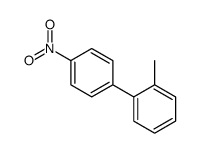
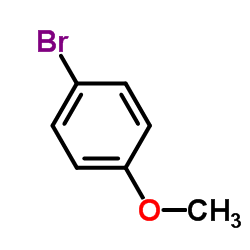
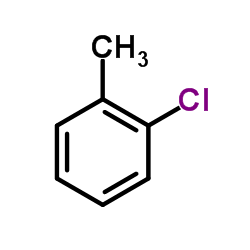
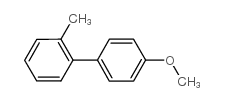
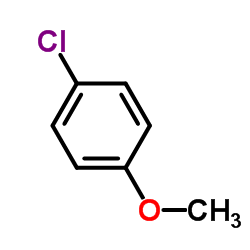
|
~84% |
|
~85% |
|
~75% |
|
~74% |
|
~87% |
|
~94% |
|
~91% |

![4'-METHOXY-[1,1'-BIPHENYL]-2-CARBONITRILE Structure](https://image.chemsrc.com/caspic/392/125610-78-8.png)