Construction of Druglike 2-Amido Benzo[d]imidazole Analogues via Desulfurative Cyclization of Thiourea Intermediate Resin on Solid-Phase
Hyun-Jeong Ryu, Seung-Ju Yang, Gee-Hyung Lee, Young-Dae Gong
Index: 10.1021/acscombsci.8b00004
Full Text: HTML
Abstract
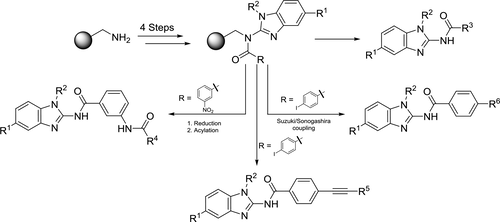
A 2-amido benzo[d]imidazole library has been constructed by solid-phase synthesis. The key step of this solid-phase synthesis involves the preparation of polymer-bound 2-amino benzo[d]imidazole resin through desulfurative cyclization of thiourea resin using 2-chloro-1,3-dimethylimidazolinium chloride and N,N-diisopropylethylamine in dichloromethane (DCM), and the resin is then functionalized by acylation at the 2-amine position to afford 2-amidobenzo[d]imidazole resin. In the case of 2-amidobenzo[d]imidazole resin having a p-I or m-NO2, the resin was further functionalized by Suzuki/Sonogashira-coupling (p-I) and reduction to the primary amine (m-NO2) followed by acylation. Finally, the functionalized 2-amido-benzo[d]imidazole resin was cleaved from the polymer support by treatment with a cocktail of trifluoroacetic acid and DCM. As a result, we obtained 2-amidobenzo[d]imidazole analogues in high yield and good purities.
|
A Parallel Approach to 7-(Hetero)arylpyrazolo[1,5-a]pyrimidi...
2018-04-06 [10.1021/acscombsci.8b00032] |
|
Solid-Phase Synthesis of β-Hydroxy Ketones Via DNA-Compatibl...
2018-04-03 [10.1021/acscombsci.8b00001] |
|
Development and Application of a Sensitive Peptide Reporter ...
2018-03-23 [10.1021/acscombsci.7b00193] |
|
A General Small-Angle X-ray Scattering-Based Screening Proto...
2018-03-23 [10.1021/acscombsci.8b00007] |
|
Aptamer-Based Paper Strip Sensor for Detecting Vibrio fische...
2018-03-22 [10.1021/acscombsci.7b00190] |