A Parallel Approach to 7-(Hetero)arylpyrazolo[1,5-a]pyrimidin-5-ones
Daniel C. Schmitt, Nootaree Niljianskul, Neal W. Sach, John I. Trujillo
Index: 10.1021/acscombsci.8b00032
Full Text: HTML
Abstract
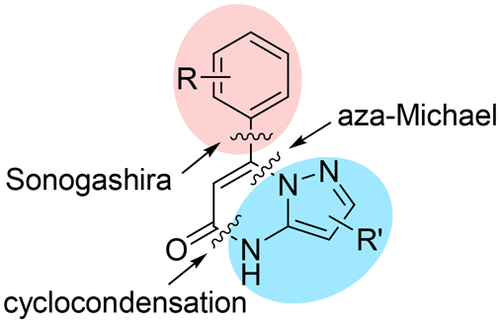
A modular, two-pot assembly of 7-arylpyrazolo[1,5-a]pyrimidones from aryl/heteroaryl halides and aminopyrazoles in library format was developed. Sonogashira coupling of aryl bromides with triethyl orthopropiolate, followed by in situ orthoester hydrolysis, provides access to β-aryl ynoates, which undergo regioselective cyclocondensation with aminopyrazoles. The ability to vary the C7 vector of 7-arylpyrazolo[1,5-a]pyrimidones in two steps using readily available (hetero)aryl halides significantly enhances synthetic access to this challenging vector.
|
Solid-Phase Synthesis of β-Hydroxy Ketones Via DNA-Compatibl...
2018-04-03 [10.1021/acscombsci.8b00001] |
|
Construction of Druglike 2-Amido Benzo[d]imidazole Analogues...
2018-03-26 [10.1021/acscombsci.8b00004] |
|
Development and Application of a Sensitive Peptide Reporter ...
2018-03-23 [10.1021/acscombsci.7b00193] |
|
A General Small-Angle X-ray Scattering-Based Screening Proto...
2018-03-23 [10.1021/acscombsci.8b00007] |
|
Aptamer-Based Paper Strip Sensor for Detecting Vibrio fische...
2018-03-22 [10.1021/acscombsci.7b00190] |