Rhodanine as a Potent Scaffold for the Development of Broad-Spectrum Metallo-β-lactamase Inhibitors
Yang Xiang, Cheng Chen, Wen-Ming Wang, Li-Wei Xu, Ke-Wu Yang, Peter Oelschlaeger, Yuan He
Index: 10.1021/acsmedchemlett.7b00548
Full Text: HTML
Abstract
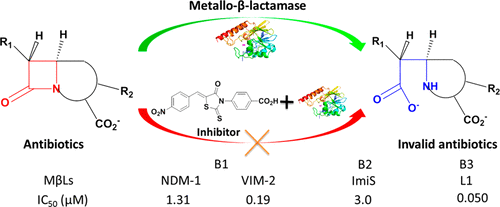
A series of rhodanines was constructed, their Z-configuration was confirmed by small molecule X-ray crystal structures, and their activity against metallo-β-lactamases (MβLs) was measured. The obtained 26 molecules and a thioenolate specifically inhibited the MβL L1 with an IC50 range of 0.02–1.7 μM, and compounds 2h–m exhibited broad-spectrum inhibition of the MβLs NDM-1, VIM-2, ImiS, and L1 with IC50 values <16 μM. All inhibitors increased the antimicrobial effect of cefazolin against E. coli cells expressing L1, resulting in a 2–8-fold reduction in MIC. Docking studies suggested that the nitro (NDM-1, CphA, and L1) or carboxyl group (VIM-2) of 2l coordinates one or two Zn(II) ions, while the N-phenyl group of the inhibitor enhances its hydrophobic interaction with MβLs. These studies demonstrate that the diaryl-substituted rhodanines are good scaffolds for the design of future broad-spectrum inhibitors of MβLs.
|
Discovery of MK-8282 as a Potent G-Protein-Coupled Receptor ...
2018-04-11 [10.1021/acsmedchemlett.8b00073] |
|
Academic Drug Development: The DRIVE Model
2018-04-11 [10.1021/acsmedchemlett.8b00124] |
|
SAR Studies of N-[2-(1H-Tetrazol-5-yl)phenyl]benzamide Deriv...
2018-04-10 [10.1021/acsmedchemlett.7b00510] |
|
Structure–Activity Relationships of Radioiodinated Benzoimid...
2018-04-10 [10.1021/acsmedchemlett.8b00092] |
|
Aminoisoxazoles as Potent Inhibitors of Tryptophan 2,3-Dioxy...
2018-04-10 [10.1021/acsmedchemlett.7b00427] |