Dual Modifications of α-Galactosylceramide Synergize to Promote Activation of Human Invariant Natural Killer T Cells and Stimulate Anti-tumor Immunity
Divya Chennamadhavuni, Noemi Alejandra Saavedra-Avila, Leandro J. Carreño, Matthew J. Guberman-Pfeffer, Pooja Arora, Tang Yongqing, Hui-Fern Koay, Dale I. Godfrey, Santosh Keshipeddy, Stewart K. Richardson, Srinivasan Sundararaj, Jae Ho Lo, Xiangshu Wen, José A. Gascón, Weiming Yuan, Jamie Rossjohn, Jérôme Le Nours, Steven A. Porcelli, Amy R. Howell
Index: 10.1016/j.chembiol.2018.02.009
Full Text: HTML
Abstract
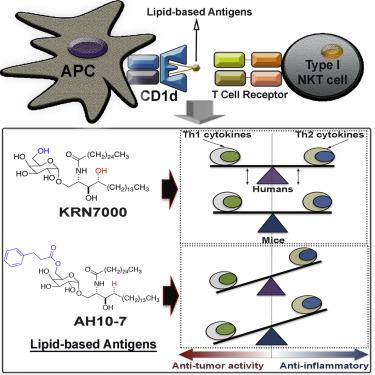
Glycosylceramides that activate CD1d-restricted invariant natural killer T (iNKT) cells have potential therapeutic applications for augmenting immune responses against cancer and infections. Previous studies using mouse models identified sphinganine variants of α-galactosylceramide as promisingiNKT cell activators that stimulate cytokine responses with a strongly proinflammatory bias. However, the activities of sphinganine variants in mice have generally not translated well to studies of humaniNKT cell responses. Here, we show that strongly proinflammatory and anti-tumoriNKT cell responses were achieved in mice by a variant of α-galactosylceramide that combines a sphinganine base with a hydrocinnamoyl ester on C6″ of the sugar. Importantly, the activities observed with this variant were largely preserved for humaniNKT cell responses. Structural andin silicomodeling studies provided a mechanistic basis for these findings and suggested basic principles for capturing useful properties of sphinganine analogs of syntheticiNKT cell activators in the design of immunotherapeutic agents.
|
Cellular Cyborgs: On the Precipice of a Drug Delivery Revolu...
2018-04-05 [10.1016/j.chembiol.2018.03.003] |
|
The Rheumatoid Arthritis-Associated Citrullinome
2018-04-05 [10.1016/j.chembiol.2018.03.002] |
|
Dependence on the Pyrimidine Biosynthetic Enzyme DHODH Is a ...
2018-04-05 [10.1016/j.chembiol.2018.03.005] |
|
The O-GlcNAc Transferase Intellectual Disability Mutation L2...
2018-03-29 [10.1016/j.chembiol.2018.03.004] |
|
Targeting Phosphopeptide Recognition by the Human BRCA1 Tand...
2018-03-29 [10.1016/j.chembiol.2018.02.012] |