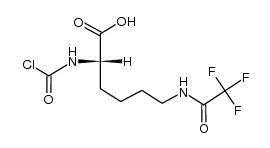
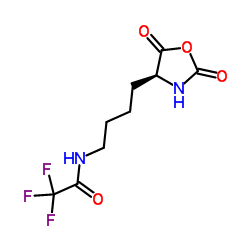
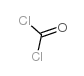
|
~94% |
|
~0% |
|
~74% |
|
~87% |
|
~% |
|
~% |
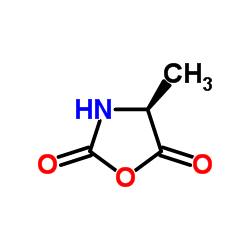
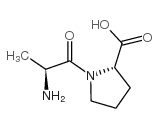
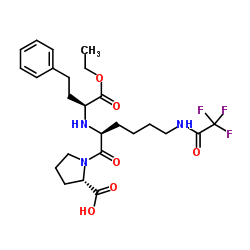
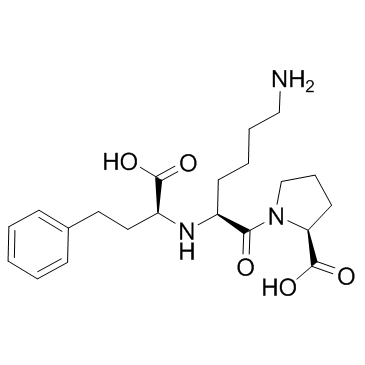
![(2S)-1-[(2S)-6-amino-2-[[(1R)-1-carboxy-3-phenylpropyl]amino]hexanoyl]pyrrolidine-2-carboxylic acid Structure](https://image.chemsrc.com/caspic/005/85955-59-5.png)