Proline
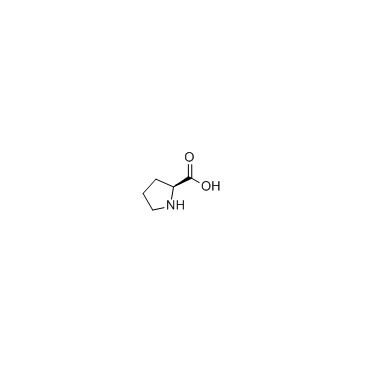
Proline structure
|
Common Name | Proline | ||
|---|---|---|---|---|
| CAS Number | 147-85-3 | Molecular Weight | 115.131 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 252.2±33.0 °C at 760 mmHg | |
| Molecular Formula | C5H9NO2 | Melting Point | 228 °C (dec.)(lit.) | |
| MSDS | Chinese USA | Flash Point | 106.3±25.4 °C | |
Use of ProlineL-Proline is one of the twenty amino acids used in living organisms as the building blocks of proteins. |
| Name | L-proline |
|---|---|
| Synonym | More Synonyms |
| Description | L-Proline is one of the twenty amino acids used in living organisms as the building blocks of proteins. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 252.2±33.0 °C at 760 mmHg |
| Melting Point | 228 °C (dec.)(lit.) |
| Molecular Formula | C5H9NO2 |
| Molecular Weight | 115.131 |
| Flash Point | 106.3±25.4 °C |
| Exact Mass | 115.063332 |
| PSA | 49.33000 |
| LogP | -0.57 |
| Vapour Pressure | 0.0±1.1 mmHg at 25°C |
| Index of Refraction | 1.487 |
| Water Solubility | soluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATAMUTATION DATA
|
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Hazard Codes | Xi:Irritant |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | TW3584000 |
| HS Code | 2933990090 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Mechanism of chemical activation of sodium chloride in the presence of amino acids.
Food Chem. 166 , 301-8, (2014) Sodium chloride has been shown to promote chlorination of glycerol during thermal processing. However, the detailed mechanism of this reaction is not well understood. Preliminary experiments have indi... |
|
|
Metabolic network capacity of Escherichia coli for Krebs cycle-dependent proline hydroxylation.
Microb. Cell Fact. 14 , 108, (2015) Understanding the metabolism of the microbial host is essential for the development and optimization of whole-cell based biocatalytic processes, as it dictates production efficiency. This is especiall... |
|
|
Injectable microcarriers as human mesenchymal stem cell support and their application for cartilage and degenerated intervertebral disc repair.
Eur. Cell. Mater. 29 , 70-80; discujssion 80-1, (2015) Degeneration of the intervertebral disc (IVD) is a progressive and chronic process, and the high incidence of discogenic disorders calls for new therapeutic approaches, such as cell-based therapies us... |
| (-)-Proline |
| BYL719 |
| Proline |
| (-)-(S)-proline |
| (2S)-proline |
| pyrrolidine-2-carbonic acid |
| (2S)-2-Pyrrolidinecarboxylic acid |
| (S)-2-Carboxypyrrolidine |
| (-)-2-Pyrrolidinecarboxylic acid |
| 2-Pyrrolidinecarboxylic acid |
| (−)-proline |
| (S)-proline |
| EINECS 210-189-3 |
| 2-Pyrrolidinecarboxylicacid |
| L(-)-Proline |
| (2S)-pyrrolidine-2-carboxylic acid |
| MFCD00064318 |
| (−)-2-pyrrolidinecarboxylic acid |
| (−)-(S)-proline |
| (S)-(-)-Pyrrolidine-2-carboxylic acid |
| L-a-Pyrrolidinecarboxylic Acid |
| (S)-(-)-Proline |
| (l)-proline |
| l-Pro |
| QCR-1 |
| (S)-Pyrrolidine-2-carboxylic acid,L-Proline |
| EINECS 205-702-2 |
| L-(-)-proline |
| L-pyrrolidine-2-carboxylic acid |
| (S)-Pyrrolidine-2-carboxylic acid |
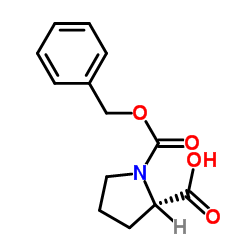 CAS#:1148-11-4
CAS#:1148-11-4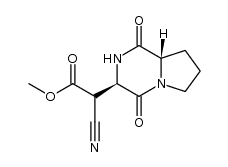 CAS#:1319113-56-8
CAS#:1319113-56-8 CAS#:15761-39-4
CAS#:15761-39-4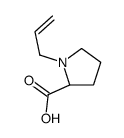 CAS#:610299-77-9
CAS#:610299-77-9 CAS#:344-25-2
CAS#:344-25-2 CAS#:439912-48-8
CAS#:439912-48-8 CAS#:110637-44-0
CAS#:110637-44-0 CAS#:91237-84-2
CAS#:91237-84-2 CAS#:7531-52-4
CAS#:7531-52-4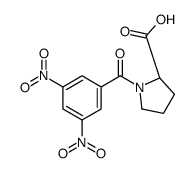 CAS#:103238-71-7
CAS#:103238-71-7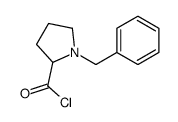 CAS#:105099-19-2
CAS#:105099-19-2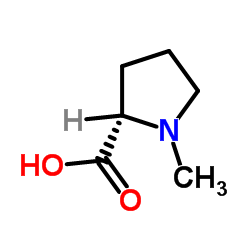 CAS#:475-11-6
CAS#:475-11-6 CAS#:36976-98-4
CAS#:36976-98-4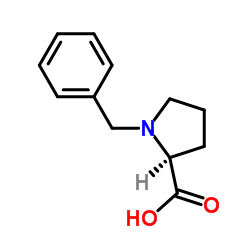 CAS#:31795-93-4
CAS#:31795-93-4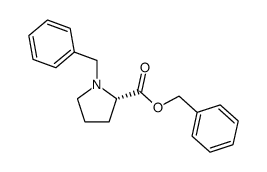 CAS#:83528-04-5
CAS#:83528-04-5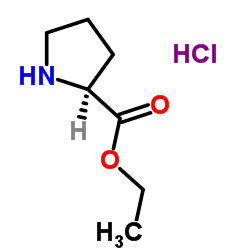 CAS#:33305-75-8
CAS#:33305-75-8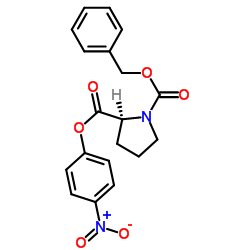 CAS#:3304-59-4
CAS#:3304-59-4![1,3-diazabicyclo[3.3.0]octane-2,4-dione structure](https://image.chemsrc.com/caspic/240/5768-79-6.png) CAS#:5768-79-6
CAS#:5768-79-6
