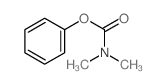
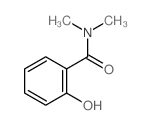
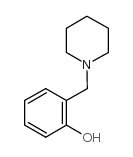
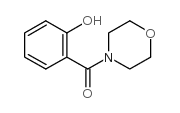
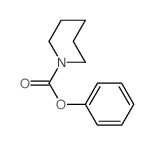
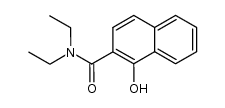
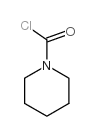
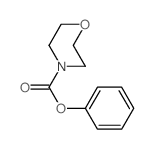
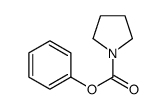
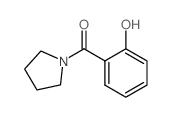
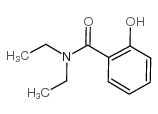
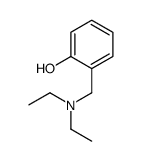
|
~72% |
|
~73% |
|
~77% |
|
~77% |
|
~78% |
|
~90% |
|
~% |
|
~82% |
|
~74% |
|
~72% |