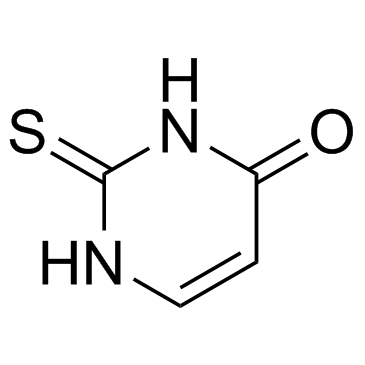
|
~76% |
|
~84% |
|
~98% |
|
~81% |
|
~96% |



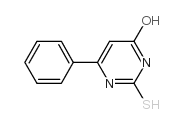
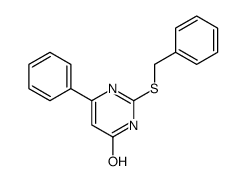
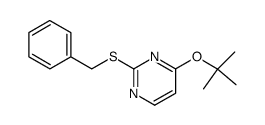
![4(3H)-Pyrimidinone,2-[(phenylmethyl)thio] Structure](https://image.chemsrc.com/caspic/213/31167-21-2.png)