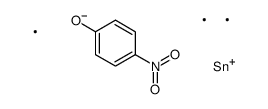
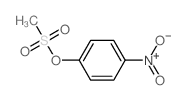
|
~% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~% |
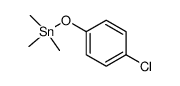
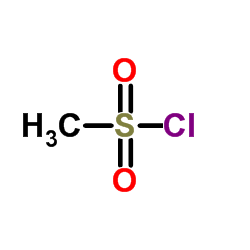
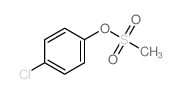
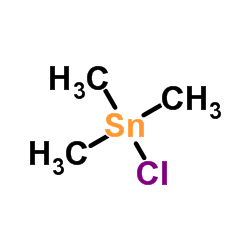
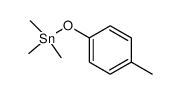

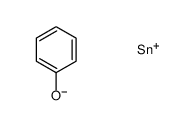
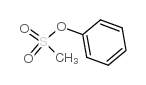
![4-Methoxyphenyl mesylate, 4-[(Methylsulphonyl)oxy]anisole Structure](https://image.chemsrc.com/caspic/187/19013-30-0.png)