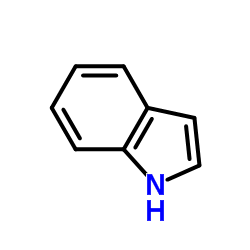
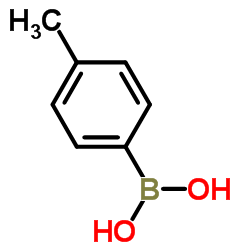
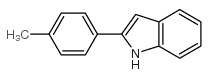
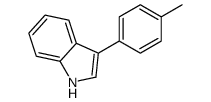
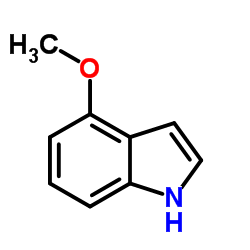
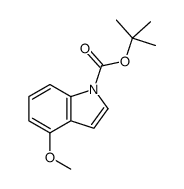
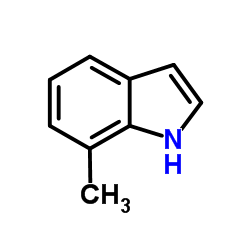
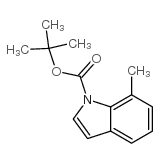
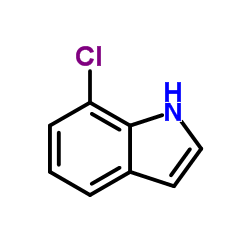
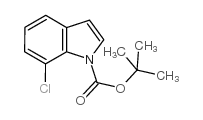
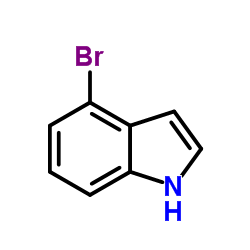
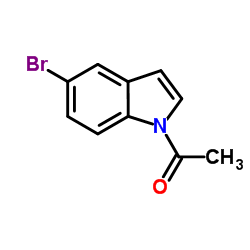
|
~9% |
|
~70% |
|
~99% |
|
~97% |
|
~94% |
|
~98% |