4-Tolylboronic acid
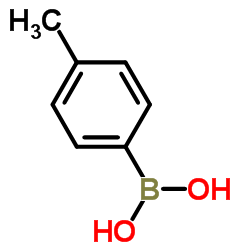
4-Tolylboronic acid structure
|
Common Name | 4-Tolylboronic acid | ||
|---|---|---|---|---|
| CAS Number | 5720-05-8 | Molecular Weight | 135.956 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 275.2±33.0 °C at 760 mmHg | |
| Molecular Formula | C7H9BO2 | Melting Point | 256-263 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 120.2±25.4 °C | |
Use of 4-Tolylboronic acid4-Tolueneboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
| Name | 4-Methylphenylboronic Acid |
|---|---|
| Synonym | More Synonyms |
| Description | 4-Tolueneboronic acid is a biochemical reagent that can be used as a biological material or organic compound for life science related research. |
|---|---|
| Related Catalog |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 275.2±33.0 °C at 760 mmHg |
| Melting Point | 256-263 °C(lit.) |
| Molecular Formula | C7H9BO2 |
| Molecular Weight | 135.956 |
| Flash Point | 120.2±25.4 °C |
| Exact Mass | 136.069565 |
| PSA | 12.03000 |
| LogP | 2.05 |
| Vapour Pressure | 0.0±0.6 mmHg at 25°C |
| Index of Refraction | 1.528 |
| InChIKey | BIWQNIMLAISTBV-UHFFFAOYSA-N |
| SMILES | Cc1ccc(B(O)O)cc1 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| Personal Protective Equipment | dust mask type N95 (US);Eyeshields;Gloves |
|---|---|
| Hazard Codes | Xi:Irritant; |
| Risk Phrases | R36/37/38 |
| Safety Phrases | S26-S36-S37/39 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| RTECS | XS7400000 |
| HS Code | 2931900090 |
| Precursor 10 | |
|---|---|
| DownStream 10 | |
| HS Code | 2931900090 |
|---|---|
| Summary | 2931900090. other organo-inorganic compounds. VAT:17.0%. Tax rebate rate:13.0%. Supervision conditions:AB(certificate of inspection for goods inward,certificate of inspection for goods outward). MFN tariff:6.5%. General tariff:30.0% |
|
Carbonic anhydrase inhibitors. Inhibition of the fungal beta-carbonic anhydrases from Candida albicans and Cryptococcus neoformans with boronic acids.
Bioorg. Med. Chem. Lett. 19 , 2642-5, (2009) Inhibition of the beta-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of aromatic, arylalkenyl- and arylalky... |
|
|
Direct palladium(II)-catalyzed synthesis of arylamidines from aryltrifluoroborates.
Org. Lett. 9th ed., 14 , 2394-2397, (2012) A fast and convenient synthesis of arylamidines starting from readily available potassium aryltrifluoroborates and cyanamides is reported. The coupling was achieved by Pd(II)-catalysis in a one step 2... |
|
|
Ruthenium(0)-catalyzed sp3 C-H bond arylation of benzylic amines using arylboronates.
Org. Lett. 7th ed., 14 , 1930-1933, (2012) A Ru-catalyzed direct arylation of benzylic sp(3) carbons of acyclic amines with arylboronates is reported. This highly regioselective and efficient transformation can be performed with various combin... |
| (R)-N-methyl-1-phenylethanamine |
| (4-Methylphenyl)boronic acid |
| ((1R)-1-phenylethyl)methylamine |
| Boronic acid, B-(4-methylphenyl)- |
| Benzenemethanamine, N,α-dimethyl-, (αR)- |
| 4-Tolylboronic acid |
| MFCD00039138 |
| 4-Methylphenylboronic acid |
| (1R)-N-Methyl-1-phenylethanamine |
| (R)-N-Methyl-α-phenylethylamine |
| EINECS 202-766-3 |
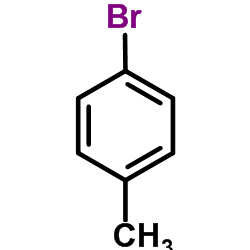 CAS#:106-38-7
CAS#:106-38-7 CAS#:624-31-7
CAS#:624-31-7 CAS#:29540-83-8
CAS#:29540-83-8 CAS#:121-43-7
CAS#:121-43-7 CAS#:106-43-4
CAS#:106-43-4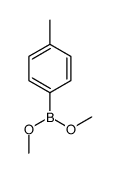 CAS#:40881-45-6
CAS#:40881-45-6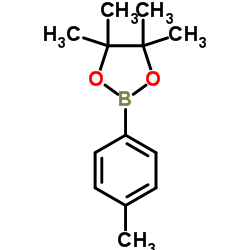 CAS#:195062-57-8
CAS#:195062-57-8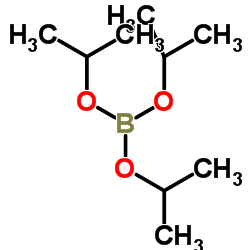 CAS#:5419-55-6
CAS#:5419-55-6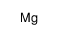 CAS#:7439-95-4
CAS#:7439-95-4 CAS#:688-74-4
CAS#:688-74-4 CAS#:10551-32-3
CAS#:10551-32-3 CAS#:108695-61-0
CAS#:108695-61-0 CAS#:503536-74-1
CAS#:503536-74-1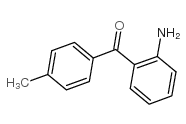 CAS#:36192-63-9
CAS#:36192-63-9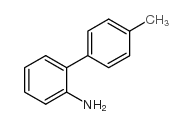 CAS#:1204-43-9
CAS#:1204-43-9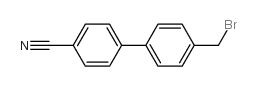 CAS#:50670-51-4
CAS#:50670-51-4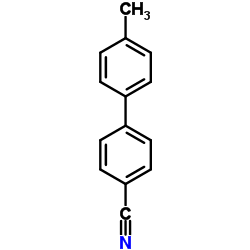 CAS#:50670-50-3
CAS#:50670-50-3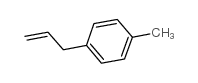 CAS#:3333-13-9
CAS#:3333-13-9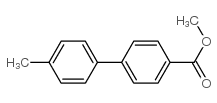 CAS#:49742-56-5
CAS#:49742-56-5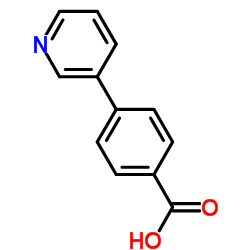 CAS#:4385-75-5
CAS#:4385-75-5
