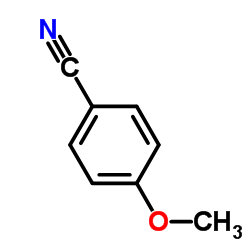
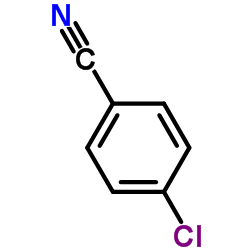
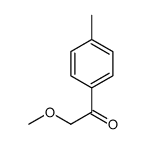
|
~77% |
|
~71% |
|
~73% |
|
~81% |
|
~79% |
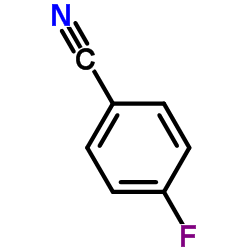
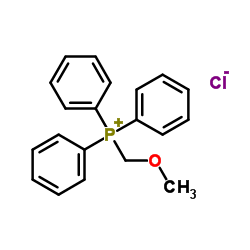
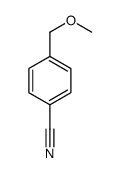
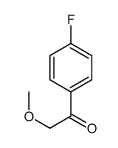
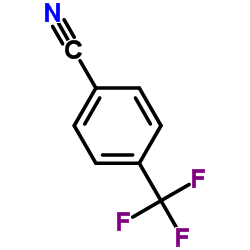
![2-Methoxy-1-[4-(trifluoromethyl)phenyl]ethanone Structure](https://image.chemsrc.com/caspic/249/26771-69-7.png)