|
~74% |
|
~% |
|
~% |
|
~% |
|
~88% |
|
~% |
|
~% |
|
~84% |
|
~% |
|
~% |

![1-nitro-4-[(E)-2-nitroprop-1-enyl]benzene Structure](https://image.chemsrc.com/caspic/188/4231-16-7.png)
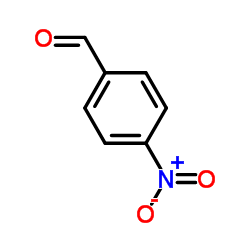
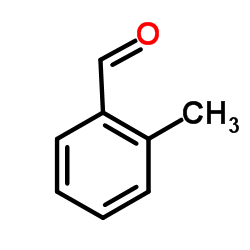
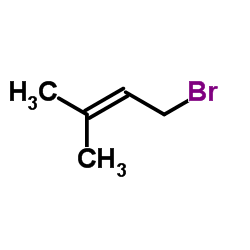
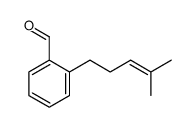
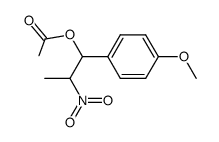
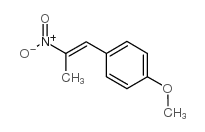

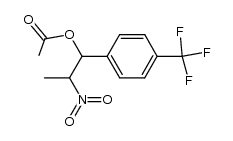
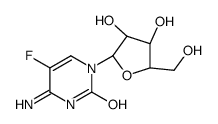
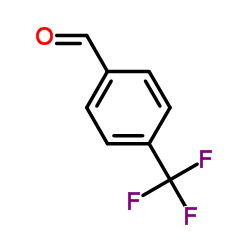
![2-Nitro-1-[4-(trifluoromethyl)phenyl]propan-1-ol Structure](https://www.chemsrc.com/extcaspic/110/21886-16-8.png)