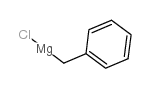
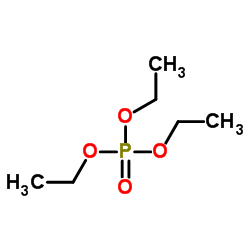
|
~% |
|
~70% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~92% |
|
~% |
|
~% |
|
~% |
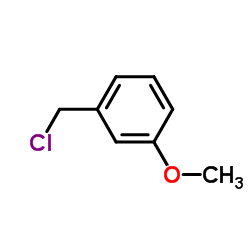
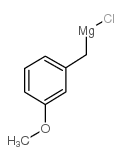

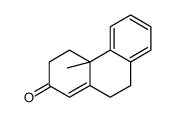
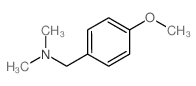

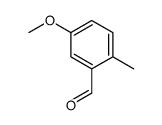
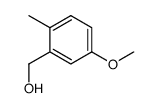
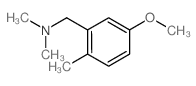
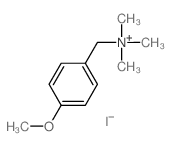
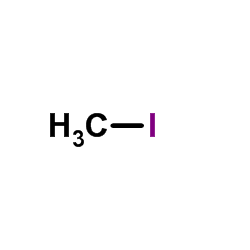
![4-methoxy-2-[2-(2-methoxyphenyl)ethyl]-1-methylbenzene Structure](https://image.chemsrc.com/caspic/236/61582-78-3.png)
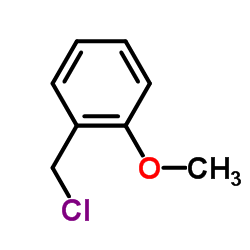
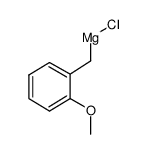
![4-methoxy-2-[2-(4-methoxyphenyl)ethyl]-1-methylbenzene Structure](https://image.chemsrc.com/caspic/198/61582-79-4.png)
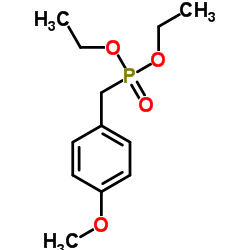
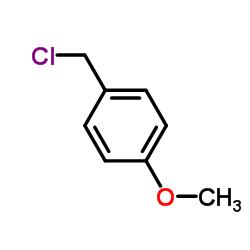
![4-methoxy-2-[2-(3-methoxyphenyl)ethyl]-1-methylbenzene Structure](https://image.chemsrc.com/caspic/343/61582-80-7.png)