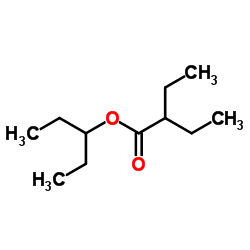
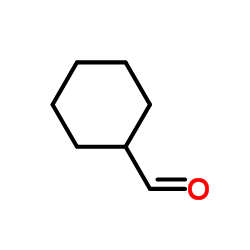
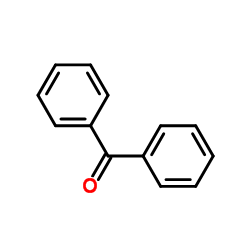
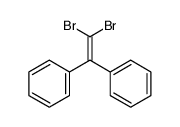
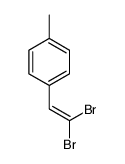
|
~88% |
|
~76% |
|
~86% |
|
~80% |