|
~58% |
|
~% |
|
~% |
|
~% |
|
~% |
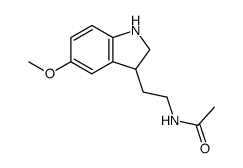
![N-[2-(1-hydroxy-5-methoxyindol-3-yl)ethyl]acetamide Structure](https://image.chemsrc.com/caspic/236/180910-62-7.png)
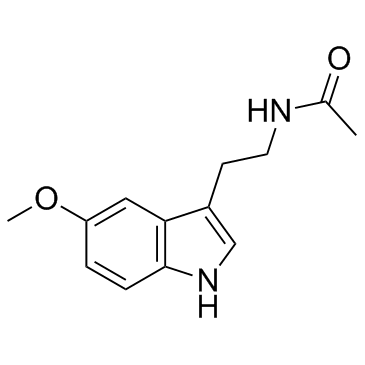
![methyl N-[2-(1-hydroxyindol-3-yl)ethyl]carbamate Structure](https://image.chemsrc.com/caspic/405/180910-53-6.png)
