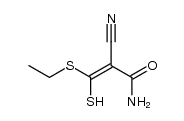
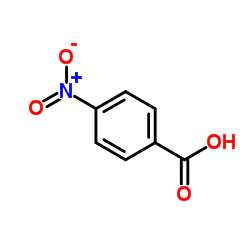
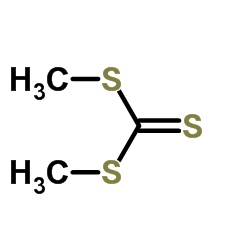
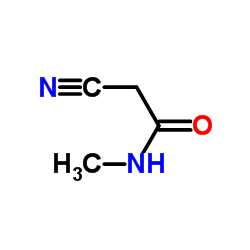
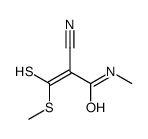
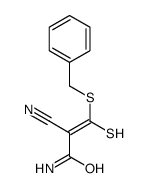
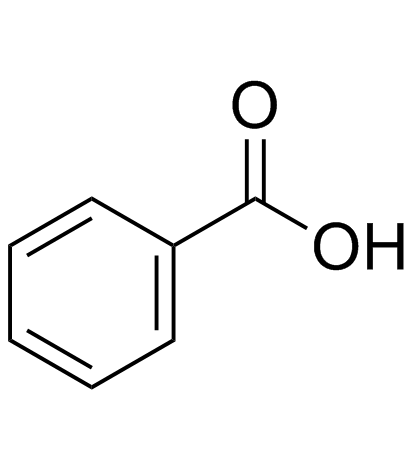
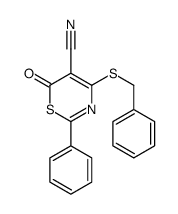
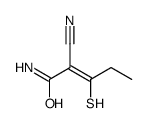
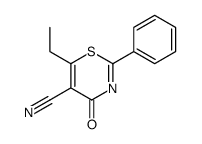
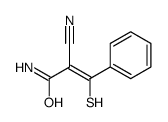
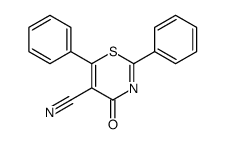
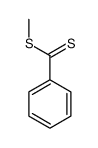
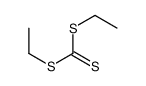
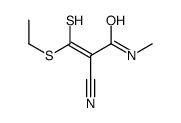
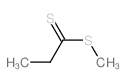
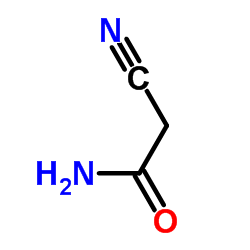
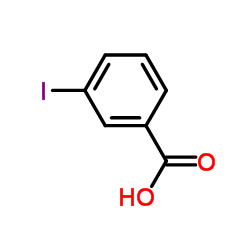
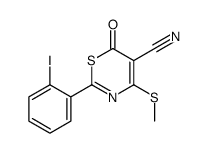
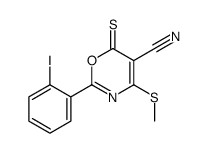
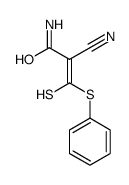
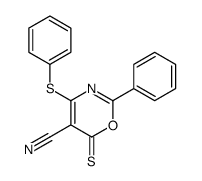
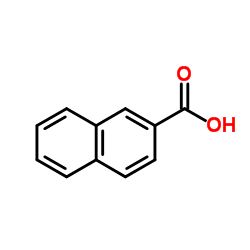
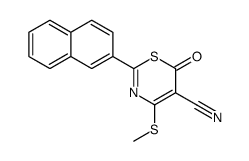
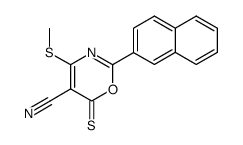
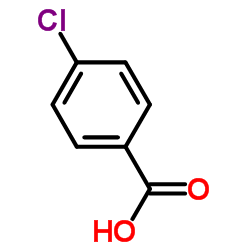
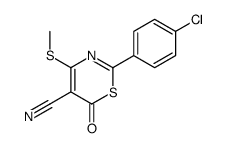
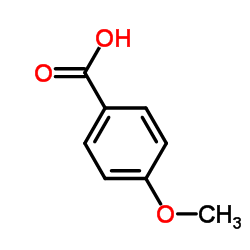
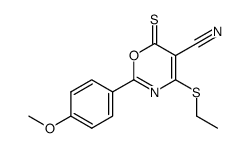
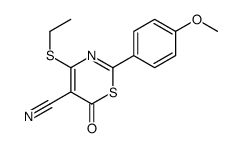
|
~30% |
|
~% |
|
~68% |
|
~50% |
|
~59% |
|
~% |
|
~33% |
|
~% |
|
~70% |
|
~79% |
|
~14% |
|
~22% |
|
~50% |
|
~58% |
|
~38% |
|
~27% |
|
~% |