|
~33% |
|
~0% |
|
~0% |
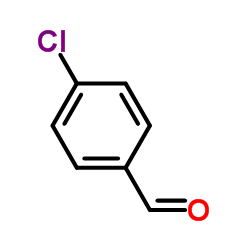
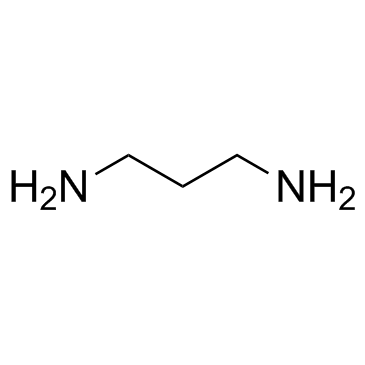
![1-(4-chlorophenyl)-N-[3-[(4-chlorophenyl)methylideneamino]propyl]methanimine Structure](https://image.chemsrc.com/caspic/335/7147-21-9.png)
![6-(4-chlorophenyl)-1,5-diazabicyclo[3.1.0]hexane Structure](https://image.chemsrc.com/caspic/413/329350-83-6.png)

![1,3-Propanediamine,N1,N3-bis[(4-methoxyphenyl)methylene] Structure](https://image.chemsrc.com/caspic/410/6958-31-2.png)
![6-(4-Methoxyphenyl)-1,5-diazabicyclo[3.1.0]hexane Structure](https://image.chemsrc.com/caspic/177/245415-93-4.png)