| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Glycerol
CAS:56-81-5 |
|
 |
Chloroform
CAS:67-66-3 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Phenol
CAS:108-95-2 |
|
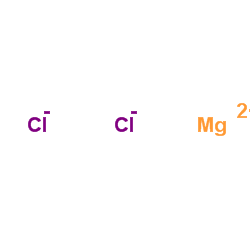 |
Magnesium choride
CAS:7786-30-3 |
|
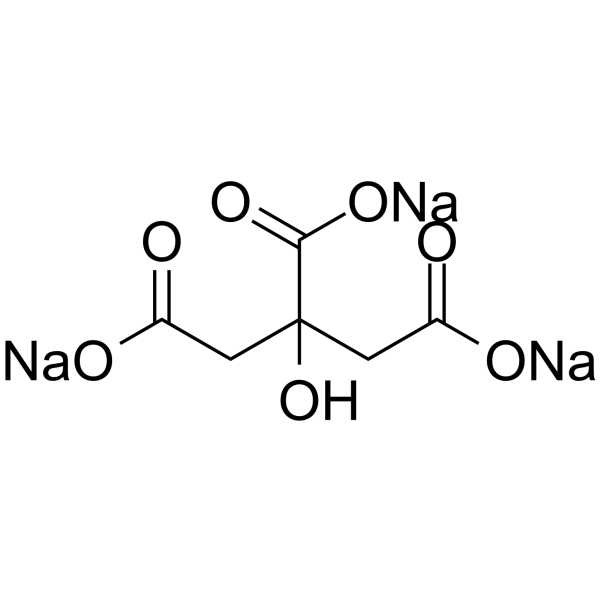 |
Sodium citrate
CAS:68-04-2 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
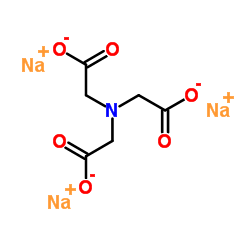 |
SODIUM NITRILOTRIACETATE
CAS:5064-31-3 |
|
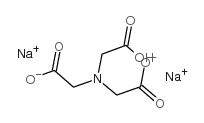 |
Disodium nitrilotriacetate
CAS:15467-20-6 |