| Structure | Name/CAS No. | Articles |
|---|---|---|
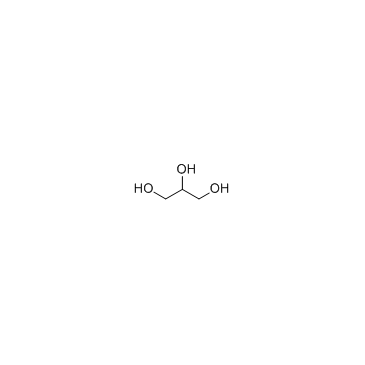 |
Glycerol
CAS:56-81-5 |
|
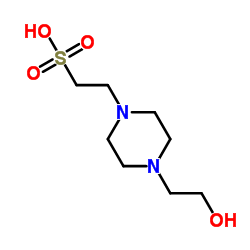 |
HEPES
CAS:7365-45-9 |
|
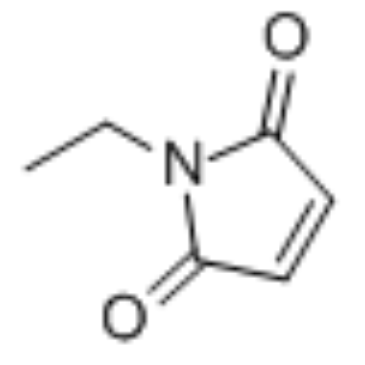 |
N-ethylmaleimide
CAS:128-53-0 |
|
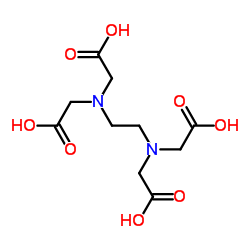 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |