| Structure | Name/CAS No. | Articles |
|---|---|---|
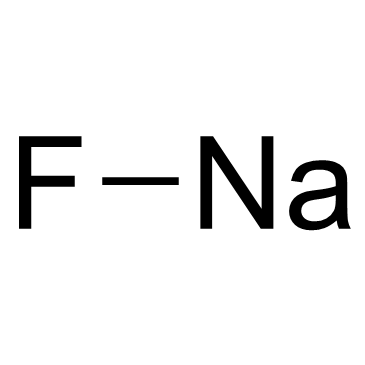 |
Sodium Fluoride
CAS:7681-49-4 |
|
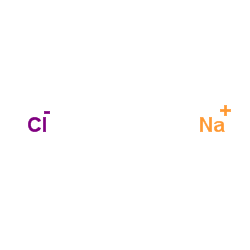 |
sodium chloride
CAS:7647-14-5 |
|
 |
sodium dodecyl sulfate
CAS:151-21-3 |
|
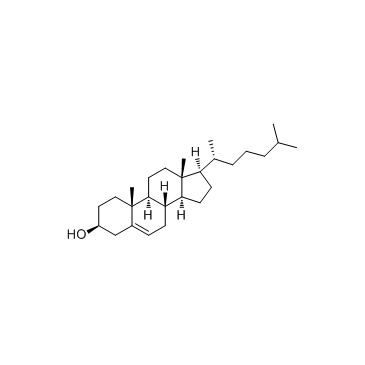 |
cholesterol
CAS:57-88-5 |
|
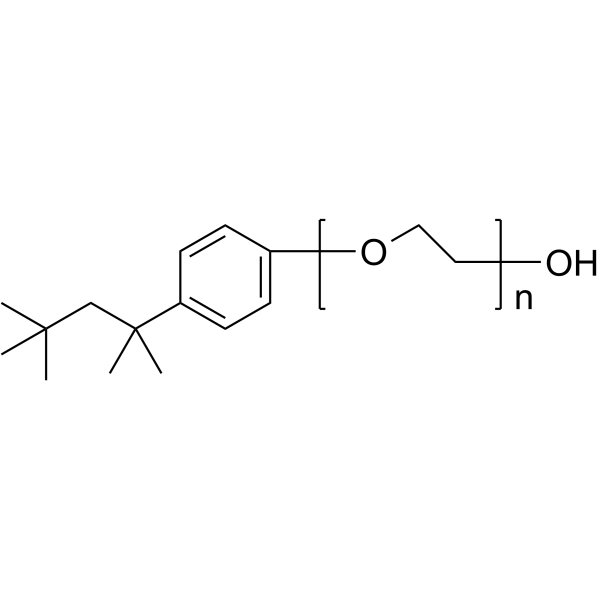 |
Triton X-100
CAS:9002-93-1 |
|
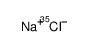 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
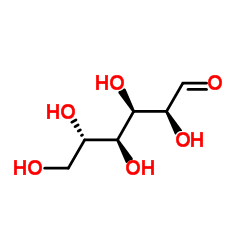 |
L-Glucose
CAS:921-60-8 |
|
 |
1,4-Dithiothreitol
CAS:16096-97-2 |
|
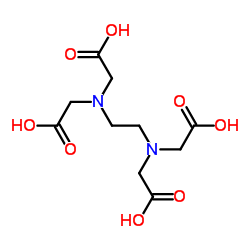 |
Ethylenediaminetetraacetic acid
CAS:60-00-4 |
|
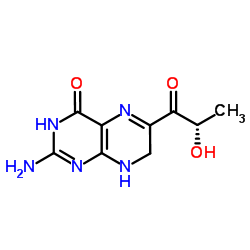 |
L-Sepiapterin
CAS:17094-01-8 |