L-Sepiapterin
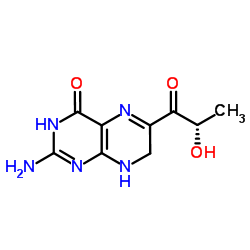
L-Sepiapterin structure
|
Common Name | L-Sepiapterin | ||
|---|---|---|---|---|
| CAS Number | 17094-01-8 | Molecular Weight | 237.215 | |
| Density | 1.9±0.1 g/cm3 | Boiling Point | 448.1ºC at 760 mmHg | |
| Molecular Formula | C9H11N5O3 | Melting Point | > 275 °C (lit.) | |
| MSDS | Chinese USA | Flash Point | 224.8ºC | |
Use of L-SepiapterinL-Sepiapterin (Sepiapterin) is a precursor of the endothelial nitric oxide synthase (eNOS) cofactor tetrahydrobiopterin (BH4). L-Sepiapterin improves endothelial dysfunction in small mesenteric arteries from db/db mice, and induces angiogenesis. L-Sepiapterin inhibits cell proliferation and migration of ovarian cancer cells via down-regulation of p70S6K-dependent VEGFR-2 expression[1][2]. |
| Name | sepiapterin |
|---|---|
| Synonym | More Synonyms |
| Description | L-Sepiapterin (Sepiapterin) is a precursor of the endothelial nitric oxide synthase (eNOS) cofactor tetrahydrobiopterin (BH4). L-Sepiapterin improves endothelial dysfunction in small mesenteric arteries from db/db mice, and induces angiogenesis. L-Sepiapterin inhibits cell proliferation and migration of ovarian cancer cells via down-regulation of p70S6K-dependent VEGFR-2 expression[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Human Endogenous Metabolite |
| In Vitro | L-Sepiapterin (Sepiapterin) (0.1-10 μM; 24 hpurs) Iinduces cell proliferation in a dose-dependent manner[1]. L-Sepiapterin (1-50 μM; 20 minutes) significantly inhibits the phosphorylation of VEGF-A-induced (50 ng/ml) p70S6K[1]. L-Sepiapterin inhibits VEGF-A-induced cell proliferation and migration through NO-independent mechanism[1]. Cell Proliferation Assay[1] Cell Line: SKOV-3 cells Concentration: 0.1, 1, 10 μM Incubation Time: 24 hours Result: Induced cell proliferation in a dose-dependent manner. |
| In Vivo | Sepiapterin (10 mg/kg; p.o. (powder chow); daily for or 8 weeks) significantly improves the relaxation to Ach in small mesenteric arteries (SMA) from db/db mice[2]. Animal Model: Male C57BL/KsJ diabetic mice (db/db)[2] Dosage: 10 mg/kg Administration: P.o. (powder chow); daily for or 8 weeks Result: Significantly improved the relaxation to Ach in SMA from db/db mice. |
| References |
| Density | 1.9±0.1 g/cm3 |
|---|---|
| Boiling Point | 448.1ºC at 760 mmHg |
| Melting Point | > 275 °C (lit.) |
| Molecular Formula | C9H11N5O3 |
| Molecular Weight | 237.215 |
| Flash Point | 224.8ºC |
| Exact Mass | 237.086182 |
| PSA | 133.46000 |
| LogP | -3.93 |
| Vapour Pressure | 6.54E-10mmHg at 25°C |
| Index of Refraction | 1.822 |
| InChIKey | VPVOXUSPXFPWBN-VKHMYHEASA-N |
| SMILES | CC(O)C(=O)C1=Nc2c(nc(N)[nH]c2=O)NC1 |
| Storage condition | 20°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | 24/25 |
| RIDADR | NONH for all modes of transport |
| HS Code | 2933990090 |
| Precursor 0 | |
|---|---|
| DownStream 1 | |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Bone Morphogenic Protein 4 Mediates NOX1-Dependent eNOS Uncoupling, Endothelial Dysfunction, and COX2 Induction in Type 2 Diabetes Mellitus.
Mol. Endocrinol. 29 , 1123-33, (2015) We have recently shown that angiotensin II-mediated uncoupling of endothelial nitric oxide synthase (eNOS) contributes to endothelial dysfunction in streptozotocin-induced type 1 diabetes mellitus. Ho... |
|
|
Sulfa drugs inhibit sepiapterin reduction and chemical redox cycling by sepiapterin reductase.
J. Pharmacol. Exp. Ther. 352(3) , 529-40, (2015) Sepiapterin reductase (SPR) catalyzes the reduction of sepiapterin to dihydrobiopterin (BH2), the precursor for tetrahydrobiopterin (BH4), a cofactor critical for nitric oxide biosynthesis and alkylgl... |
|
|
Stromal cell-derived factor 2 is critical for Hsp90-dependent eNOS activation.
Sci. Signal. 8 , ra81, (2015) Endothelial nitric oxide synthase (eNOS) catalyzes the conversion of l-arginine and molecular oxygen into l-citrulline and nitric oxide (NO), a gaseous second messenger that influences cardiovascular ... |
| 2-amino-6-(2-hydroxy-propionyl)-7,8-dihydro-3H-pteridin-4-one |
| MFCD00210214 |
| 1-(2-amino-7,8-dihydro-4-hydroxy-6-pteridinyl)-2-hydroxy-1-Propanone |
| 4(3H)-Pteridinone, 2-amino-7,8-dihydro-6-[(2S)-2-hydroxy-1-oxopropyl]- |
| 2-amino-7,8-dihydro-6-[(2S)-2-hydroxy-1-oxopropyl]-4(1H)-Pteridinone |
| 2-amino-7,8-dihydro-6-[(2S)-2-hydroxy-1-oxopropyl]-4(1H)Pteridinone |
| (S)-2-amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4(1H)-Pteridinone |
| Sepiapterine |
| L-Sepiapterin |
| Sepiapterin,S-()-2-Amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4(1H)-pteridinone,L-Sepiapterin |
| 2-Amino-6-[(2S)-2-hydroxypropanoyl]-7,8-dihydro-4(1H)-pteridinone |
| Sepiapterin |
| 2-Amino-6-L-lactoyl-7,8-dihydro-3H-pteridin-4-on |
| 2-amino-6-(2-hydroxypropanoyl)-7,8-dihydro-1H-pty oeridin-4-one |
| 2-AMINO-7,8-DIHYDRO-6-(2S-HYDROXY-1-OXOPROPYL)-4(1H)-PTERIDINONE |
| 2-amino-6-[(2S)-2-hydroxypropanoyl]-7,8-dihydro-1H-pteridin-4-one |
| 2-Amino-6-[(2S)-2-hydroxypropanoyl]-7,8-dihydropteridin-4(3H)-one |
| S-(−)-2-Amino-7,8-dihydro-6-(2-hydroxy-1-oxopropyl)-4(1H)-pteridinone |
| S(-)-2-AMINO-7,8-DIHYDRO-6-(2-HYDROXY-1-OXOPROPYL)-4(1H)-PTERIDINONE |
| 2-amino-6-L-lactoyl-7,8-dihydro-3H-pteridin-4-one |
 CAS#:490-79-9
CAS#:490-79-9