| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
 |
Dimethyl sulfoxide
CAS:67-68-5 |
|
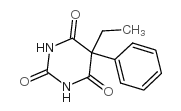 |
phenobarbital
CAS:50-06-6 |
|
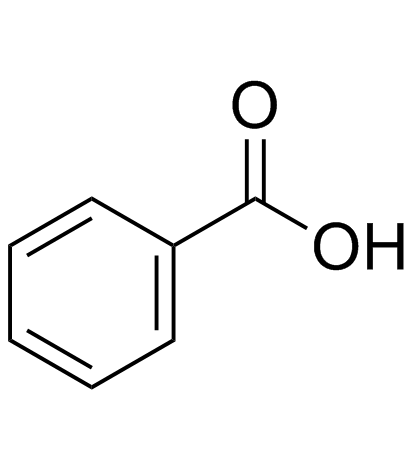 |
benzoic acid
CAS:65-85-0 |
|
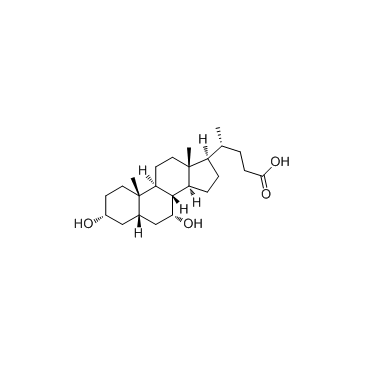 |
Chenodeoxycholic acid
CAS:474-25-9 |
|
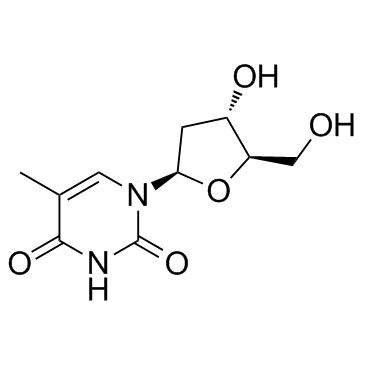 |
Thymidine
CAS:50-89-5 |
|
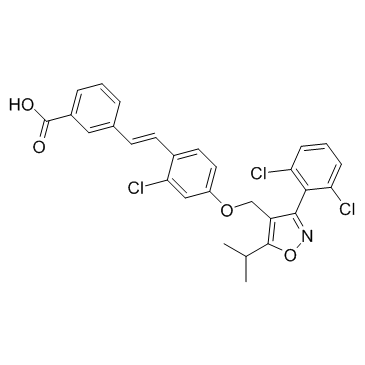 |
GW4064
CAS:278779-30-9 |
|
 |
Chlormethine hydrochloride
CAS:55-86-7 |
|
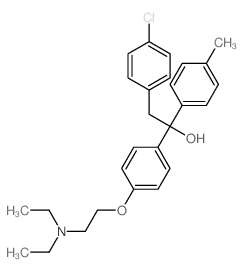 |
Triparanol
CAS:78-41-1 |
|
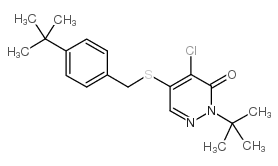 |
Pyridaben
CAS:96489-71-3 |