| Structure | Name/CAS No. | Articles |
|---|---|---|
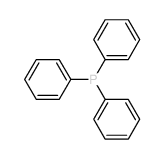 |
Triphenylphosphine resin
CAS:39319-11-4 |
|
 |
Thiophane
CAS:110-01-0 |
|
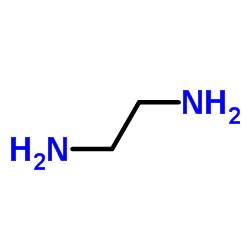 |
1,2-Ethanediamine
CAS:107-15-3 |
|
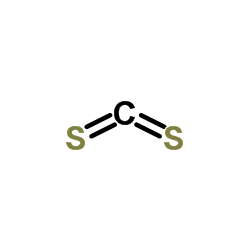 |
Carbon disulphide
CAS:75-15-0 |
|
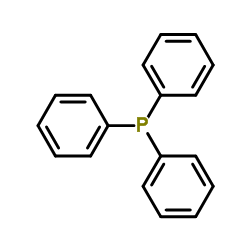 |
Triphenylphosphine
CAS:603-35-0 |