| Structure | Name/CAS No. | Articles |
|---|---|---|
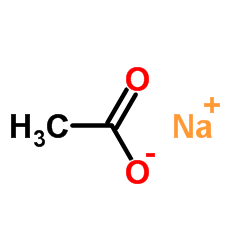 |
Sodium acetate
CAS:127-09-3 |
|
 |
sodium chloride
CAS:7647-14-5 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
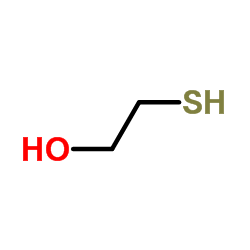 |
mercaptoethanol
CAS:60-24-2 |
|
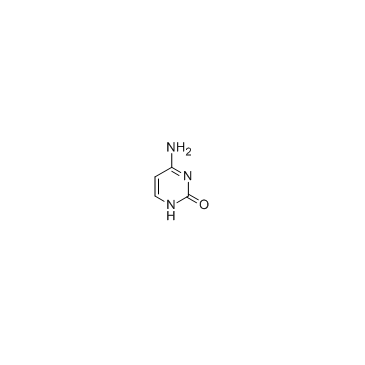 |
cytosine
CAS:71-30-7 |
|
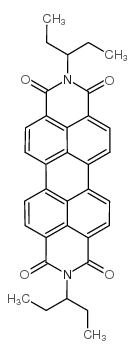 |
2,9-Di(pent-3-yl)anthra2,1,9-def:6,5,10-d'e'f'diisoquinoline-1,3,8,10-tetrone
CAS:110590-81-3 |