| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Carbon
CAS:7440-44-0 |
|
 |
Gold
CAS:7440-57-5 |
|
 |
acrolein
CAS:107-02-8 |
|
 |
Cinnamic aldehyde
CAS:14371-10-9 |
|
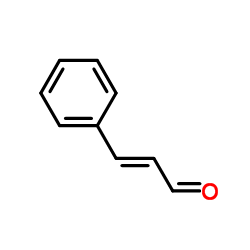 |
Cinnamaldehyde
CAS:104-55-2 |