| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Dipicolinic acid
CAS:499-83-2 |
|
 |
Isopropanol
CAS:67-63-0 |
|
 |
Ethanol
CAS:64-17-5 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
Tetrabutylammonium bromide
CAS:1643-19-2 |
|
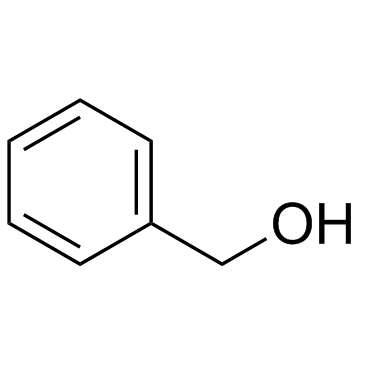 |
Benzyl alcohol
CAS:100-51-6 |
|
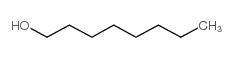 |
1-Octanol
CAS:111-87-5 |
|
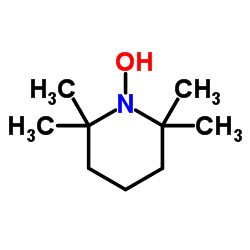 |
2,2,6,6-Tetramethylpiperidinooxy
CAS:2564-83-2 |
|
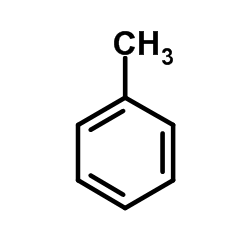 |
Toluene
CAS:108-88-3 |
|
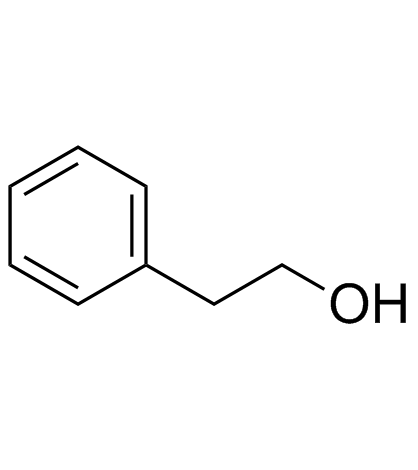 |
Phenethyl alcohol
CAS:60-12-8 |