| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
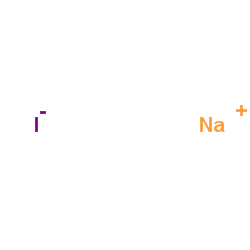 |
Sodium iodide
CAS:7681-82-5 |
|
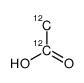 |
acetic acid
CAS:1173022-32-6 |
|
 |
acetic acid
CAS:64-19-7 |
|
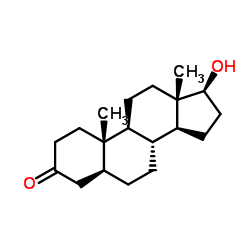 |
Stanolone
CAS:521-18-6 |