Stanolone
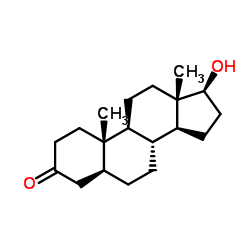
Stanolone structure
|
Common Name | Stanolone | ||
|---|---|---|---|---|
| CAS Number | 521-18-6 | Molecular Weight | 290.440 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 413.1±38.0 °C at 760 mmHg | |
| Molecular Formula | C19H30O2 | Melting Point | 178-183 °C | |
| MSDS | Chinese USA | Flash Point | 176.4±19.4 °C | |
| Symbol |



GHS02, GHS06, GHS08 |
Signal Word | Danger | |
| Name | 17β-hydroxy-5α-androstan-3-one |
|---|---|
| Synonym | More Synonyms |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 413.1±38.0 °C at 760 mmHg |
| Melting Point | 178-183 °C |
| Molecular Formula | C19H30O2 |
| Molecular Weight | 290.440 |
| Flash Point | 176.4±19.4 °C |
| Exact Mass | 290.224579 |
| PSA | 37.30000 |
| LogP | 3.75 |
| Vapour Pressure | 0.0±2.2 mmHg at 25°C |
| Index of Refraction | 1.536 |
| InChIKey | NVKAWKQGWWIWPM-ABEVXSGRSA-N |
| SMILES | CC12CCC3C(CCC4CC(=O)CCC43C)C1CCC2O |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS06, GHS08 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H301 + H311 + H331-H370 |
| Precautionary Statements | P210-P280-P302 + P352 + P312-P304 + P340 + P312-P370 + P378-P403 + P235 |
| Personal Protective Equipment | Eyeshields;full-face particle respirator type N100 (US);Gloves;respirator cartridge type N100 (US);type P1 (EN143) respirator filter;type P3 (EN 143) respirator cartridges |
| Hazard Codes | Xn |
| Risk Phrases | R61 |
| Safety Phrases | S53-S36/37/39-S45 |
| RIDADR | UN1230 - class 3 - PG 2 - Methanol, solution |
| WGK Germany | 3 |
| RTECS | BV8052000 |
| Packaging Group | II |
| Hazard Class | 6.1 |
| Precursor 8 | |
|---|---|
| DownStream 10 | |
|
Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis.
Cancer Res. 75(6) , 1102-12, (2015) The translation initiation factor eIF4E is an oncogene that is commonly overexpressed in primary breast cancers and metastases. In this article, we report that a pharmacologic inhibitor of eIF4E funct... |
|
|
Aptamer-based polyvalent ligands for regulated cell attachment on the hydrogel surface.
Biomacromolecules 16(4) , 1382-9, (2015) Natural biomolecules are often used to functionalize materials to achieve desired cell-material interactions. However, their applications can be limited owing to denaturation during the material funct... |
|
|
Co-ordinated brain and craniofacial development depend upon Patched1/XIAP regulation of cell survival.
Hum. Mol. Genet. 24(3) , 698-713, (2015) Congenital brain and craniofacial defects often occur together as a consequence of their developmental dependency on common progenitor tissue interactions and signaling pathways during embryogenesis. ... |
| Stanolone |
| EINECS 208-307-3 |
| Anaboleen |
| 17β-Hydroxy-5α-androstane-3-one |
| Androlone |
| dihydrotestosterone |
| Anabolex |
| (5a,17b)-17-Hydroxyandrostan-3-one |
| 5α-Dihydrotestosterone |
| Stanorone |
| 5α dihydrotestosterone |
| Androstanolone |
| DHT |
| [14C]-Dihydrotestosterone |
| Androstan-3-one, 17-hydroxy-, (5α,17β)- |
| 5α-Androstan-3-one, 17β-hydroxy- |
| [3H]-Dihydrotestosterone |
| 17beta-hydroxy-5alpha-androstan-3-one |
| Andractim |
| 17β-hydroxy-5α-androstan-3-one |
| Androstan-17b-ol-3-one |
| Stanaprol |
| 4-dihydrotestosterone |
| Neodrol |
| (5α,17β)-17-Hydroxyandrostan-3-one |
| 5a-Dihydrotestosterone |
| Protona |
| Stanolon |
| Anaprotin |
| MFCD00003667 |
 CAS#:58701-44-3
CAS#:58701-44-3![(5S,8R,9S,10S,13S,14S,17S)-10,13-dimethyl-17-((triethylsilyl)oxy)tetradecahydro-1H-cyclopenta[a]phenanthren-3(2H)-one Structure](https://image.chemsrc.com/caspic/212/361336-13-2.png) CAS#:361336-13-2
CAS#:361336-13-2 CAS#:846-46-8
CAS#:846-46-8 CAS#:1046-35-1
CAS#:1046-35-1![Androstan-3-one, 17-[(2-methoxyethoxy)methoxy]-, (17β) Structure](https://image.chemsrc.com/caspic/359/91475-95-5.png) CAS#:91475-95-5
CAS#:91475-95-5 CAS#:58-22-0
CAS#:58-22-0![3,17β-bis-[2,3,5,6-tetrafluoro-4-(trifluoromethyl)phenoxy]-5α-androst-2-ene Structure](https://image.chemsrc.com/caspic/319/112251-17-9.png) CAS#:112251-17-9
CAS#:112251-17-9![(3R,5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl (3,5-di-tert-butyl-4-oxocyclohexa-2,5-dien-1-ylidene)azinate Structure](https://image.chemsrc.com/caspic/241/75958-96-2.png) CAS#:75958-96-2
CAS#:75958-96-2 CAS#:1827-75-4
CAS#:1827-75-4 CAS#:481-29-8
CAS#:481-29-8 CAS#:53-41-8
CAS#:53-41-8 CAS#:571-20-0
CAS#:571-20-0![(3R,5S,8R,9S,10S,13S,14S,17S)-10,13-dimethylspiro[1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopenta[a]phenanthrene-3,2'-oxirane]-17-ol structure](https://image.chemsrc.com/caspic/290/2384-24-9.png) CAS#:2384-24-9
CAS#:2384-24-9 CAS#:1852-53-5
CAS#:1852-53-5![(5S,8R,9S,10R,13S,14S)-10,13-dimethyl-5,6,7,8,9,11,12,14,15,16-decahydro-4H-cyclopenta[a]phenanthrene-3,17-dione structure](https://image.chemsrc.com/caspic/493/571-40-4.png) CAS#:571-40-4
CAS#:571-40-4 CAS#:27261-27-4
CAS#:27261-27-4 CAS#:121209-70-9
CAS#:121209-70-9