| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Permethrin
CAS:52645-53-1 |
|
 |
(3-Phenoxyphenyl)methanol
CAS:13826-35-2 |
|
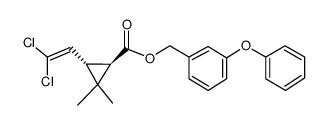 |
trans-permethrin
CAS:61949-77-7 |
|
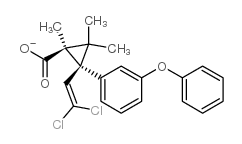 |
cis-Permethrin Standard
CAS:61949-76-6 |