Permethrin

Permethrin structure
|
Common Name | Permethrin | ||
|---|---|---|---|---|
| CAS Number | 52645-53-1 | Molecular Weight | 391.288 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 465.9±45.0 °C at 760 mmHg | |
| Molecular Formula | C21H20Cl2O3 | Melting Point | 34-35°C | |
| MSDS | Chinese USA | Flash Point | 159.4±27.7 °C | |
| Symbol |



GHS02, GHS07, GHS09 |
Signal Word | Danger | |
Use of PermethrinPermethrin (NRDC-143) is an insecticide, acaricide, and insect repellent; functions as a neurotoxin, affecting neuron membranes by prolonging sodium channel activation. |
| Name | permethrin |
|---|---|
| Synonym | More Synonyms |
| Description | Permethrin (NRDC-143) is an insecticide, acaricide, and insect repellent; functions as a neurotoxin, affecting neuron membranes by prolonging sodium channel activation. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 465.9±45.0 °C at 760 mmHg |
| Melting Point | 34-35°C |
| Molecular Formula | C21H20Cl2O3 |
| Molecular Weight | 391.288 |
| Flash Point | 159.4±27.7 °C |
| Exact Mass | 390.078949 |
| PSA | 35.53000 |
| LogP | 7.15 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.616 |
| InChIKey | RLLPVAHGXHCWKJ-UHFFFAOYSA-N |
| SMILES | CC1(C)C(C=C(Cl)Cl)C1C(=O)OCc1cccc(Oc2ccccc2)c1 |
| Stability | Stable. Incompatible with strong oxidising agents. |
| Water Solubility | insoluble |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| Symbol |



GHS02, GHS07, GHS09 |
|---|---|
| Signal Word | Danger |
| Hazard Statements | H225-H302-H312-H319-H332-H411 |
| Precautionary Statements | P210-P273-P280-P305 + P351 + P338 |
| Personal Protective Equipment | Eyeshields;Faceshields;full-face respirator (US);Gloves;multi-purpose combination respirator cartridge (US);type ABEK (EN14387) respirator filter |
| Hazard Codes | Xn: Harmful;N: Dangerous for the environment;T: Toxic;F: Flammable; |
| Risk Phrases | R20/22 |
| Safety Phrases | S13-S24-S36/37/39-S60-S61-S45-S36/37-S16-S7-S26 |
| RIDADR | UN1230 3/PG 2 |
| RTECS | GZ1255000 |
| HS Code | 2916209022 |
|
~76% 
Permethrin CAS#:52645-53-1 |
| Literature: Sagami Chemical Research Center Patent: US4214097 A1, 1980 ; |
|
~93% 
Permethrin CAS#:52645-53-1 |
| Literature: Bayer Aktiengesellschaft Patent: US4225704 A1, 1980 ; |
|
~95% 
Permethrin CAS#:52645-53-1 |
| Literature: Nakatsuji, Hidefumi; Morita, Jun-Ichi; Misaki, Tomonori; Tanabe, Yoo Advanced Synthesis and Catalysis, 2006 , vol. 348, # 15 p. 2057 - 2062 |
|
~% 
Permethrin CAS#:52645-53-1 |
| Literature: Kuraray Co., Ltd. Patent: US4113968 A1, 1978 ; |
|
~% 
Permethrin CAS#:52645-53-1 |
| Literature: Sumitomo Chemical Company, Limited Patent: US6441220 B1, 2002 ; |
|
Detail
|
| Literature: Sagami Chemical ResearchCenter Patent: US4500733 A1, 1985 ; |
|
~% 
Permethrin CAS#:52645-53-1 |
| Literature: Sumitomo Chemical Company, Limited Patent: US6531626 B1, 2003 ; |
|
~% 
Permethrin CAS#:52645-53-1 |
| Literature: Sumitomo Chemical Company, Limited Patent: US6531626 B1, 2003 ; |
| Precursor 9 | |
|---|---|
| DownStream 2 | |
| HS Code | 2916209022 |
|---|
|
Cheminformatics analysis of assertions mined from literature that describe drug-induced liver injury in different species.
Chem. Res. Toxicol. 23 , 171-83, (2010) Drug-induced liver injury is one of the main causes of drug attrition. The ability to predict the liver effects of drug candidates from their chemical structures is critical to help guide experimental... |
|
|
Translating clinical findings into knowledge in drug safety evaluation--drug induced liver injury prediction system (DILIps).
J. Sci. Ind. Res. 65(10) , 808, (2006) Drug-induced liver injury (DILI) is a significant concern in drug development due to the poor concordance between preclinical and clinical findings of liver toxicity. We hypothesized that the DILI typ... |
|
|
Developing structure-activity relationships for the prediction of hepatotoxicity.
Chem. Res. Toxicol. 23 , 1215-22, (2010) Drug-induced liver injury is a major issue of concern and has led to the withdrawal of a significant number of marketed drugs. An understanding of structure-activity relationships (SARs) of chemicals ... |
| Anomethrin N |
| 3-(Phenoxyphenyl)methyl (±)-cis,trans-3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| kudos |
| Ambushfog |
| Cyclopropanecarboxylic acid, 3-(2,2-dichloroethenyl)-2,2-dimethyl-, (3-phenoxyphenyl)methyl ester |
| Peregin |
| Stomozan |
| Perigen |
| Kestrel |
| Pounce |
| Peregin W |
| SBP 15131TEC |
| 3-Phenoxybenzyl-3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropancarboxylat |
| Chinetrin |
| Outflank |
| L3TJ A1 A1 BVO1R COR&& C1UYGG |
| Permanone 80 |
| ICI-PP 557 |
| Picket G |
| 1RS,3SR)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Exmin |
| EXPAR |
| 3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Eksmin |
| Kafil |
| Stomoxin |
| 3-phenoxybenzyl (1RS,3RS |
| Indothrin |
| Cosair |
| BW-21-Z |
| Quamlin |
| Ambush |
| Kavil |
| Ridect Pour-On |
| Talcord |
| (3-phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Outflank-stockade |
| 3-Dichloroacetyl-4-hydroxy-1-methyl-2-oxo-1,2-dihydroquinoline |
| Insorbcid MP |
| MFCD00041809 |
| Efmethrin |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Lyclear |
| Ecsumin |
| Perigen W |
| Diffusil H |
| m-Phenoxybenzyl (±)-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| [3-(phenyloxy)phenyl]methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Permethrin |
| (±)-3-Phenoxybenzyl 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichlorethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Imperator |
| Coopex |
| m-Phenoxybenzyl (±)-cis,trans-3-(2,2-Dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Stomoxi |
| Permethrin (cis/trans) |
| Acticin |
| Picket |
| Permasect-25EC |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid (3-phenoxyphenyl)methyl ester |
| Bematin 987 |
| Permasect |
| SBP-1513TEC |
| Pynosect |
| Exsmin |
| 3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropanecarboxylic acid, (3-phenoxyphenyl)methyl ester |
| MP79 |
| Permethrin (isomers) |
| 3-phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2- dimethylcyclopropanecarboxylate |
| (3-Phenoxyphenyl)methyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate |
| LE 79-519 |
| (3-phenoxyphenyl)methyl (1Ξ,3Ξ)-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropane-1-carboxylate |
| Antiborer 3768 |
| Kaleait |
| 3-Phenoxybenzyl 3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| EINECS 258-067-9 |
| Stomoxin P |
| Dragnet FT |
| Ectiban |
| Mitin BC |
| (3-Phenoxyphenyl)methyl (±)-cis,trans-3-(2,2-dichloroethenyl)-2,2-dimethylcyclopropanecarboxylate |
| Ipitox |
| Pramex |
| 3-Phenoxybenzyl (1RS)-cis-trans-3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropanecarboxylate |
| Corsair |
| NIX |
| Dragnet |
| 3-Phenoxybenzyl 2,2-dimethyl-3-(2,2-dichlorovinyl)cyclopropanecarboxylate |
| Cooper |
| Perthrine |
| SBP-3246 |
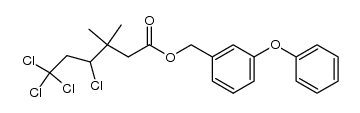
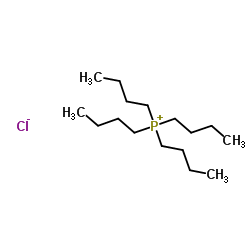

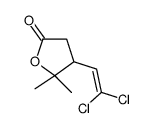
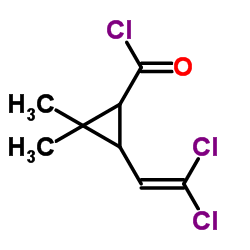


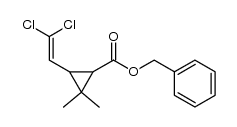
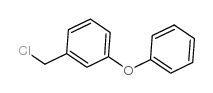
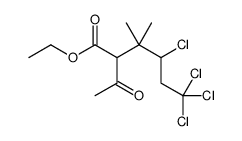
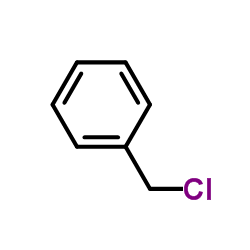
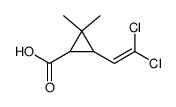 CAS#:55701-05-8
CAS#:55701-05-8
