| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Acetone
CAS:67-64-1 |
|
 |
Methanol
CAS:67-56-1 |
|
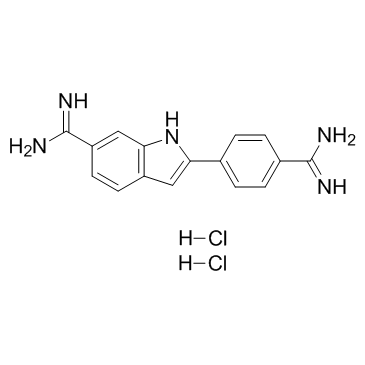 |
4',6-Diamidino-2-phenylindole dihydrochloride
CAS:28718-90-3 |
|
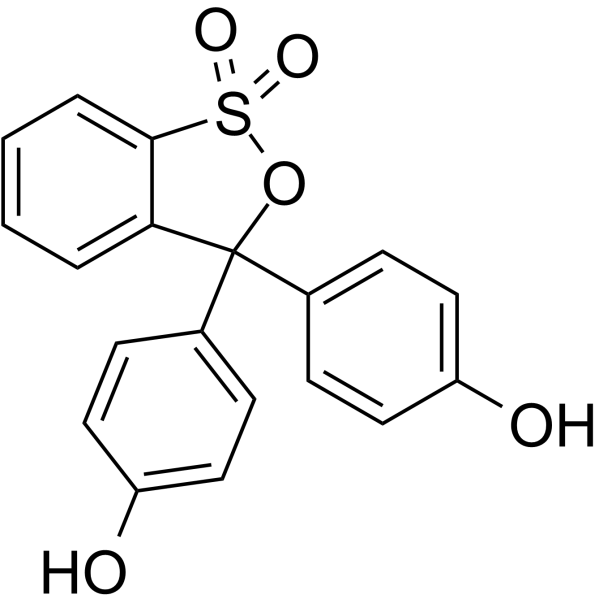 |
Phenol red
CAS:143-74-8 |
|
 |
HUMAN VEGF165
CAS:127464-60-2 |
|
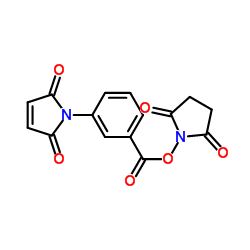 |
MBS Crosslinker
CAS:58626-38-3 |