| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Isopropanol
CAS:67-63-0 |
|
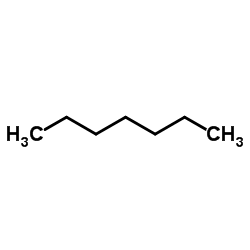 |
Heptane
CAS:142-82-5 |
|
 |
ethyl acetate
CAS:141-78-6 |
|
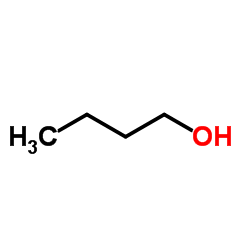 |
Butanol
CAS:71-36-3 |
|
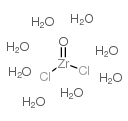 |
Zirconyl chloride octahydrate
CAS:13520-92-8 |
|
 |
4-Nitrophenol
CAS:100-02-7 |
|
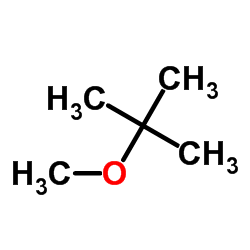 |
Methyl tert-butyl ether
CAS:1634-04-4 |
|
 |
Benzaldehyde
CAS:100-52-7 |
|
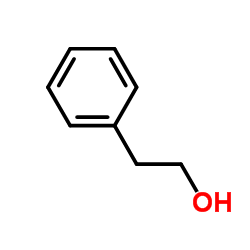 |
Methylbenzylalcohol
CAS:98-85-1 |
|
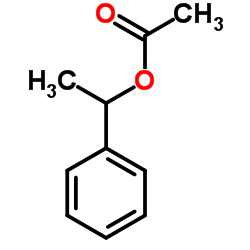 |
1-Phenylethyl acetate
CAS:93-92-5 |