| Structure | Name/CAS No. | Articles |
|---|---|---|
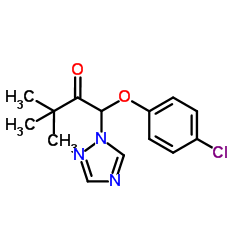 |
Triadimefon
CAS:43121-43-3 |
|
 |
Epoxiconazole
CAS:133855-98-8 |
|
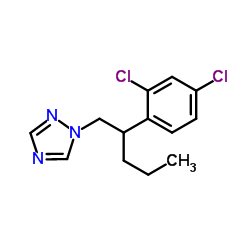 |
penconazole
CAS:66246-88-6 |
|
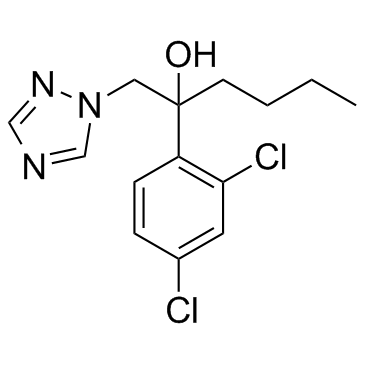 |
Hexaconazole
CAS:79983-71-4 |