| Structure | Name/CAS No. | Articles |
|---|---|---|
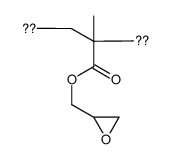 |
poly(glycidyl methacrylate) macromolecule
CAS:25067-05-4 |
|
 |
4-aminosalicylic acid
CAS:65-49-6 |
|
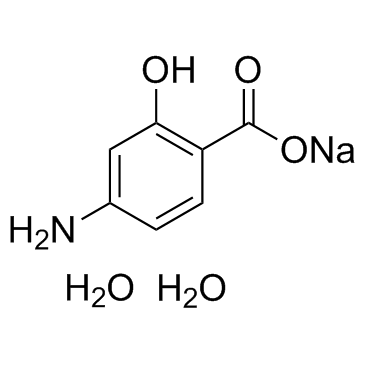 |
Sodium 4-aminosalicylate dihydrate
CAS:6018-19-5 |