| Structure | Name/CAS No. | Articles |
|---|---|---|
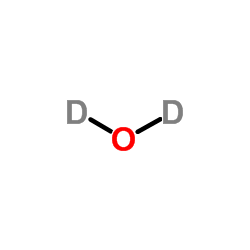 |
Heavy water
CAS:7789-20-0 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
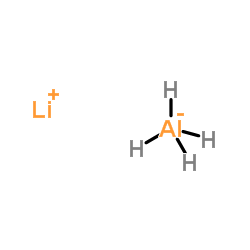 |
Lithium Aluminium Hydride
CAS:16853-85-3 |
|
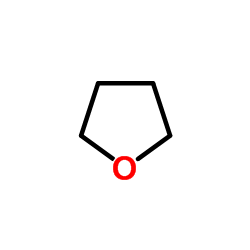 |
thf
CAS:109-99-9 |
|
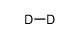 |
deuterium
CAS:7782-39-0 |
|
 |
benzene
CAS:71-43-2 |
|
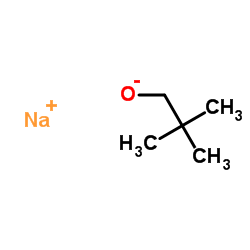 |
Sodium tert-pentoxide
CAS:14593-46-5 |
|
 |
Calcium
CAS:7789-78-8 |
|
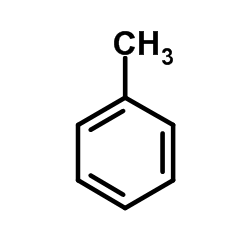 |
Toluene
CAS:108-88-3 |