Archives of Biochemistry and Biophysics
1984-07-01
Formation of novel nitrofuran metabolites in xanthine oxidase-catalyzed reduction of methyl 5-nitro-2-furoate.
H Yamada, K Tatsumi, Y Kawazoe, H Yoshimura
Index: Arch. Biochem. Biophys. 232(1) , 234-9, (1984)
Full Text: HTML
Abstract
After methyl 5-nitro-2-furoate was incubated with milk xanthine oxidase, three reduction products were isolated from the incubation mixture. Among them, two reduction products were new types of nitrofuran metabolites, i.e., metabolites 1 and 2 were identified as the dihydroxyhydrazine derivative (1,2-dihydroxy-1,2-di(5-methoxycarbonyl-2-furyl)hydrazine) and the hydroxylaminofuran derivative (methyl 5-hydroxylamino-2-furoate), respectively. Metabolite 3 was also identified as the aminofuran derivative (methyl 5-amino-2-furoate) by comparison with a synthetic sample.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
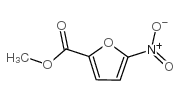 |
methyl 5-nitro-2-furoate
CAS:1874-23-3 |
C6H5NO5 |
Related Articles:
More...
|
Reduction of methyl 5-nitro-2-furoate by xanthine oxidase: a...
1982-07-01 [Chem. Pharm. Bull. 30(7) , 2647-50, (1982)] |
|
Yaws CL.
[The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals 2nd ed.,, (2015), 97] |
|
Magic matrices for ionization in mass spectrometry. Trimpin ...
[Int. J. Mass Spectrom. 377 , 532-545, (2015)] |
|
Synthesis and antimicrobial activity of methyl 5-nitro-3,4-d...
[Chem. Pharm. Bull. 29(3) , 635-645, (1981)] |
|
Syntheses with 5-Nitro-2-furonitrile. Sherman WR and Esch AV...
[J. Med. Chem. 8(1) , 25-28, (1965)] |