| Structure | Name/CAS No. | Articles |
|---|---|---|
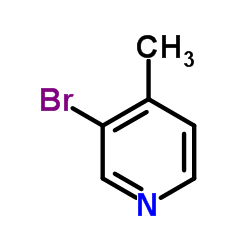 |
3-Bromo-4-methylpyridine
CAS:3430-22-6 |
|
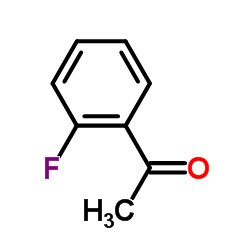 |
1-(2-Fluorophenyl)ethanone
CAS:445-27-2 |