From evidence assessments to coverage decisions?: the case example of glinides in Germany.
Julia Kreis, Reinhard Busse
Index: Health Policy 104(1) , 27-31, (2012)
Full Text: HTML
Abstract
In Germany, coverage decisions in the statutory health insurance (SHI) system are based on the principles of evidence-based medicine. Recently, an evidence assessment by the Institute for Quality and Efficiency in Health Care (IQWiG) of the oral antidiabetics of the glinide class showed that their long-term benefit is not proven. Accordingly, the responsible Federal Joint Committee (G-BA) decided to exclude glinides from prescription in the SHI system. This was, however, objected to by the Ministry of Health, which is charged with legal supervision. We use this case to illustrate the path from evidence assessments to coverage decisions in Germany against the background of the latest health reform, which has changed the legal requirements for evidence assessments and the ensuing coverage decisions.Copyright © 2011 Elsevier Ireland Ltd. All rights reserved.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
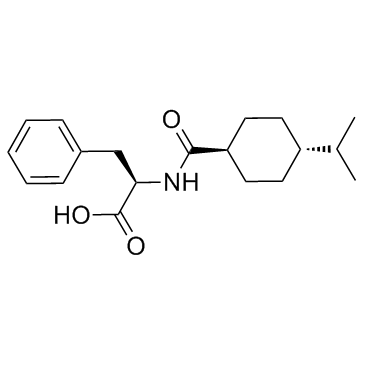 |
Nateglinide
CAS:105816-04-4 |
C19H27NO3 |
|
Formulation and in vitro evaluation of nateglinide microsphe...
2013-11-01 [Pak. J. Pharm. Sci. 26(6) , 1229-35, (2013)] |
|
Nateglinide--current and future role in the treatment of pat...
2005-10-01 [Int. J. Clin. Pract. 59(10) , 1218-28, (2005)] |
|
Efficacy and safety of mitiglinide versus nateglinide in new...
2012-02-01 [Diabetes Obes. Metab. 14(2) , 187-9, (2012)] |
|
Nateglinide (Starlix): update on a new antidiabetic agent.
2003-01-01 [Int. J. Clin. Pract. 57(6) , 535-41, (2003)] |
|
II. Technological approaches to improve the dissolution beha...
2013-09-15 [Int. J. Pharm. 454(1) , 568-72, (2013)] |