A randomized, double-blinded, crossover pilot study assessing the effect of nabilone on spasticity in persons with spinal cord injury.
Sepideh Pooyania, Karen Ethans, Tony Szturm, Alan Casey, Daryl Perry
Index: Arch. Phys. Med. Rehabil. 91(5) , 703-7, (2010)
Full Text: HTML
Abstract
To determine whether nabilone, a synthetic cannabinoid, alleviates spasticity in people with spinal cord injury (SCI).A double-blind, placebo-controlled crossover study.Outpatient rehabilitation clinics.We recruited volunteers (N=12) with SCI and spasticity. One subject, a paraplegic man, dropped out of the study because of an unrelated cause. Eleven subjects completed the study; all subjects were men with an average age of 42.36 years; 6 of them were persons with tetraplegia, and 5 were persons with paraplegia.The subjects received either nabilone or placebo during the first 4-week period (0.5mg once a day with option to increase to 0.5mg twice a day), and then outcome measures were assessed. After a 2-week washout, subjects were crossed over to the opposite arm.The primary outcome was the Ashworth Scale for spasticity in the most involved muscle group, in either the upper or lower extremities, chosen by the subject and clinician. The secondary outcomes included the sum of the Ashworth Scale in 8 muscle groups of each side of the body measured by the clinician; Spasm Frequency Scale and visual analog scale, reported by the subject; Wartenberg Pendulum Test, in order to quantify severity of spasticity; and the Clinician's and Subject's Global Impression of Change.One subject dropped out during the placebo arm because of an unrelated urinary stricture, and 11 subjects completed the study. There was a significant decrease on active treatment for the Ashworth in the most involved muscle (mean difference +/- SD, .909+/-.85; P=.003), as well as the total Ashworth score (P=.001). There was no significant difference in other measures. Side effects were mild and tolerable.Nabilone may be beneficial to reduce spasticity in people with SCI. We recommend a larger trial with a more prolonged treatment period and an option to slowly increase the dosage further.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
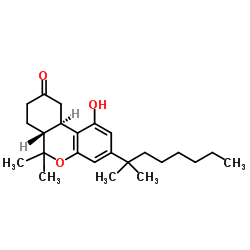 |
(−)-nabilone
CAS:51022-71-0 |
C24H36O3 |
|
Oral nabilone capsules in the treatment of chemotherapy-indu...
2008-01-01 [Expert Opin. Investig. Drugs 17(1) , 85-95, (2008)] |
|
An open-label comparison of nabilone and gabapentin as adjuv...
2011-01-01 [Pain Pract. 11(4) , 353-68, (2011)] |
|
Subjective, cognitive and cardiovascular dose-effect profile...
2013-09-01 [Addict. Biol. 18(5) , 872-81, (2013)] |
|
Cannabinoids in the treatment of chemotherapy-induced nausea...
2012-04-01 [J. Natl. Compr. Canc. Netw. 10(4) , 487-92, (2012)] |
|
Analgesic and antihyperalgesic effects of nabilone on experi...
2008-04-01 [Curr. Med. Res. Opin. 24(4) , 1017-24, (2008)] |